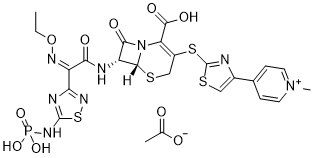
Ceftaroline fosamil (formerly also known as TAK-599 and PPI-0903) is a new generation of antibiotic belonging to the cephalosporin class with activity against Gram-positive pathogens, including methicillin-resistant Staphylococcus aureus (MRSA). Ceftaroline fosamil retains the activity of later-generation cephalosporins having broad-spectrum activity against Gram-negative bacteria. It is currently being investigated for community-acquired pneumonia and complicated skin and skin structure infection.
Physicochemical Properties
| Molecular Formula | C22H21N8O8PS4 |
| Molecular Weight | 744.7 |
| Exact Mass | 684.01 |
| Elemental Analysis | C, 38.71; H, 3.38; N, 15.05; O, 21.48; P, 4.16; S, 17.22 |
| CAS # | 400827-46-5 |
| Related CAS # | Ceftaroline fosamil (hydrate)(acetate);400827-55-6;Ceftaroline fosamil (inner);229016-73-3;400827-46-5 |
| PubChem CID | 56841980 |
| Appearance | White to light yellow solid powder |
| LogP | -2.84 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 19 |
| Rotatable Bond Count | 11 |
| Heavy Atom Count | 47 |
| Complexity | 1240 |
| Defined Atom Stereocenter Count | 2 |
| SMILES | S1C([H])([H])C(=C(C(=O)[O-])N2C([C@]([H])([C@@]12[H])N([H])C(/C(/C1=NSC(N([H])P(=O)(O[H])O[H])=N1)=N/OC([H])([H])C([H])([H])[H])=O)=O)SC1=NC(C2C([H])=C([H])[N+](C([H])([H])[H])=C([H])C=2[H])=C([H])S1 |
| InChi Key | UGHHNQFYEVOFIV-VRDMTWHKSA-N |
| InChi Code | InChI=1S/C22H21N8O8PS4.C2H4O2/c1-3-38-26-13(16-25-21(43-28-16)27-39(35,36)37)17(31)24-14-18(32)30-15(20(33)34)12(9-40-19(14)30)42-22-23-11(8-41-22)10-4-6-29(2)7-5-10;1-2(3)4/h4-8,14,19H,3,9H2,1-2H3,(H4-,24,25,27,28,31,33,34,35,36,37);1H3,(H,3,4)/b26-13-;/t14-,19-;/m1./s1 |
| Chemical Name | 4-(2-(((6R,7R)-2-carboxy-7-((Z)-2-(ethoxyimino)-2-(5-(phosphonoamino)-1,2,4-thiadiazol-3-yl)acetamido)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl)thio)thiazol-4-yl)-1-methylpyridin-1-ium acetate |
| Synonyms | T-91825; T 91825; T91825; Teflaro; Zinforo;TAK 599; TAK599; TAK-599; PPI 0903; PP 0903; PPI-0903; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | β-lactam |
| ln Vitro | Ceftaroline (TAK-599) is a novel N-phosphono prodrug of anti-methicillin-resistant Staphylococcus aureus (MRSA) cephalosporin 2a (T-91825) that has high affinity for penicillin-binding protein (PBP) 2' (IC(50); 0.90 microg/mL) and shows potent in vitro anti-MRSA activity (MIC against MRSA N133; 1.56 microg/mL), comparable to that of vancomycin (1.56 microg/mL). [1] |
| ln Vivo |
With ED50s of 1.60–2.37 mg/kg, ceftaroline fosamil (s.c.) protects mice from experimental systemic infection caused by S. aureus N133[1]. In the blood of rats and monkeys, ceftaroline fosamil (10 mg/kg; s.c.) vanishes quickly and smoothly transforms into T-91825[1]. |
| Enzyme Assay | High-performance liquid chromatography was used to determine the concentrations of linezolid (lower detection limit, 0.1 mg/liter; coefficient of variation, <10%). Assays with vancomycin were performed by an immunoenzymatic method with a COBAS MIRA unit and EMIT reagents (detection threshold, 2.5 mg/liter; coefficient of variation, 4.1 to 6.9%). Active ceftaroline concentrations were determined by a microbiologic assay with Bacillus subtilis as the test organism and antibiotic medium 2 as the diffusion medium (lower detection limit, 0.25 mg/liter; intraday and interday variations, <10%). [2] |
| Animal Protocol |
Using the neutropenic lung infection model, 17 clinical S. aureus isolates (2 MSSA, 15 MRSA) are investigated. For a duration of 24 hours, groups of six mice are treated with Ceftaroline fosamil starting three hours after inoculation. Doses of ceftaroline fosamil are injected subcutaneously in increments of 0.2 mL. Normal saline is given to control animals in the same amounts, ways, and intervals as the treatment plans[1]. For ceftaroline, blood samples were taken from six healthy rabbits after administration of a ceftaroline acetate bolus of 10 and 30 mg/kg of body weight in order to determine the spontaneous drug kinetics. The simulation was intended to provide apparent values of pharmacokinetic parameters close to those observed in healthy volunteers after a 1-h infusion of a 600-mg dose (ca. 10 mg/kg) of ceftaroline acetate: mean half-life (t1/2), 1.57 to 2.63 h; peak concentration (Cmax), 18.96 to 21.02 mg/liter; and area under the curve (AUC), 56.08 mg·h/liter. A total dose of 58 mg/kg needed to be infused into the rabbit over a 12-h period in order to simulate the kinetics in human serum after the administration of a 10-mg/kg dose (i.e., 600 mg twice daily). For each MRSA strain, the animals were randomly assigned to either no treatment (controls), ceftaroline regimen mimicking the human dose of 10 mg/kg every 12 h (q12h) (600 mg q12h), a linezolid regimen mimicking the human dose of 10 mg/kg q12h (600 mg q12h), and vancomycin administered by a constant intravenous infusion in order to reach a steady-state 20× MIC in serum. Experimental endocarditis was induced with an inoculum of 108 CFU of S. aureus. Treatment was started 24 h after inoculation for a 4-day regimen. Aortic valve vegetations were excised, weighed, and then homogenized in 0.5 ml of saline buffer and used for quantitative cultures on agar for 24 h at 37°C. Dilutions at 10−1, 10−2, and 10−4 were prepared to eliminate potential carryover effects. To evaluate whether ceftaroline treatment could induce the selection of variants resistant in vivo, undiluted vegetation homogenates were spread on agar plates containing the active form of ceftaroline at a concentration corresponding to fourfold the MIC. Bacterial counts were determined after 48 h of incubation at 37°C.[2] |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion primarily eliminated by the kidneys (6% in feces within 48 hours). Median 20.3 L (18.3-21.6 L). Metabolism / Metabolites Ceftaroline fosamil is converted into bioactive ceftaroline in plasma by a phosphatase enzyme. Hydrolysis of the beta-lactam ring of ceftaroline occurs to form the microbiologically inactive, open-ring metabolite ceftaroline M-1. Biological Half-Life 1.60 hours (600 mg dose). TAK-599 has not only practical water solubility, but also good chemical stability in the solid state and solution. Although cephalosporin 2a (T-91825) had insufficient water solubility (2.3 mg/mL) for parenteral administration, 1 (TAK-599) showed excellent water solubility (>100 mg/mL, pH 7) as well as good chemical stability in the solid state and solution. In pharmacokinetic studies, when 1 was administered intravenously to rats and monkeys, it was rapidly converted into 2a in the blood. These results show that 1 (TAK-599) is a highly promising parenteral cephalosporin targeted for MRSA infection.[1] |
| Toxicity/Toxicokinetics |
Protein Binding approximately 20%. |
| References |
[1]. TAK-599, a novel N-phosphono type prodrug of anti-MRSA cephalosporin T-91825: synthesis, physicochemical and pharmacological properties. Bioorg Med Chem. 2003 May 29;11(11):2427-37. [2]. In vivo efficacy of ceftaroline (PPI-0903), a new broad-spectrum cephalosporin, compared with linezolid and vancomycin against methicillin-resistant and vancomycin-intermediate Staphylococcus aureus in a rabbit endocarditis model. Antimicrob Agents Chemother. 2007 Sep;51(9):3397-400. [3]. Ceftaroline fosamil, a cephalosporin derivative for the potential treatment of MRSA infection. Curr Opin Investig Drugs. 2008 Feb;9(2):201-9. |
| Additional Infomation |
Pharmacodynamics The time that unbound plasma concentration of ceftaroline exceeds the minimum inhibitory concentration (MIC) of the infecting organism has been shown to best correlate with efficacy in a neutropenic murine thigh infection model with S. aureus and S. pneumoniae. No significant effect on QTc (corrected QT interval) interval was detected at peak plasma concentration or at any other time. |
Solubility Data
| Solubility (In Vitro) | DMSO : 73~100 mg/mL ( 98.02~134.28 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (2.79 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (2.79 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (2.79 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.08 mg/mL (2.79 mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3428 mL | 6.7141 mL | 13.4282 mL | |
| 5 mM | 0.2686 mL | 1.3428 mL | 2.6856 mL | |
| 10 mM | 0.1343 mL | 0.6714 mL | 1.3428 mL |