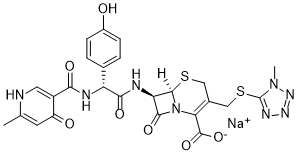
Cefpiramide sodium (also known as SM-1652, or Wy-44635) is the sodium salt of Cefpiramide which is a new ureido cephalosporin antibiotic. Cefpiramide inhibited many Pseudomonas aeruginosa resistant to carbenicillin, piperacillin, and cefotaxime, but it was less active than ceftazidime and cefsulodin. Cefpiramide inhibited staphylococci and streptococci and had appreciable activity against Streptococcus faecalis and Listeria moncytogenes. It had less activity than cefoxitin against anaerobic species. Cefpiramide inhibited permeability mutants of Escherichia coli at lower concentrations than the parent strain, suggesting an effect of entry upon its activity. Although cefpiramide was resistant to attack by most chromosomal beta-lactamases, it was hydrolyzed by the common plasmid beta-lactamases TEM and SHV and by the chromosomal Proteus vulgaris, type Ic, cephalosporinase.
Physicochemical Properties
| Molecular Formula | C25H23N8NAO7S2 |
| Molecular Weight | 634.62 |
| Exact Mass | 634.102 |
| Elemental Analysis | C, 47.31; H, 3.65; N, 17.66; Na, 3.62; O, 17.65; S, 10.11 |
| CAS # | 74849-93-7 |
| Related CAS # | Cefpiramide;70797-11-4 |
| PubChem CID | 23663969 |
| Appearance | Solid powder |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 9 |
| Heavy Atom Count | 43 |
| Complexity | 1270 |
| Defined Atom Stereocenter Count | 3 |
| SMILES | O=C(C(N12)=C(CSC3=NN=NN3C)CS[C@]2([H])[C@H](NC([C@H](NC(C4=C(O)C=C(C)N=C4)=O)C5=CC=C(O)C=C5)=O)C1=O)O.[Na] |
| InChi Key | RIWWMGQFMUUYIY-ALLHVENQSA-M |
| InChi Code | InChI=1S/C25H24N8O7S2.Na/c1-11-7-16(35)15(8-26-11)20(36)27-17(12-3-5-14(34)6-4-12)21(37)28-18-22(38)33-19(24(39)40)13(9-41-23(18)33)10-42-25-29-30-31-32(25)2;/h3-8,17-18,23,34H,9-10H2,1-2H3,(H,26,35)(H,27,36)(H,28,37)(H,39,40);/q;+1/p-1/t17-,18-,23-;/m1./s1 |
| Chemical Name | sodium;(6R,7R)-7-[[(2R)-2-(4-hydroxyphenyl)-2-[(6-methyl-4-oxo-1H-pyridine-3-carbonyl)amino]acetyl]amino]-3-[(1-methyltetrazol-5-yl)sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate |
| Synonyms | Cefpiramide Sodium; CHEBI:31377; SM-1652; UNII-137KB7GYKB; WY-44635; cefpiramide, sodium salt; SM 1652; SM-1652; Sumcefal; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: (1). Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture and light.(2). This product is not stable in solution, please use freshly prepared working solution for optimal results. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | β-lactam | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Animal Protocol |
|
||
| References |
[1]. Cefpiramide: comparative in-vitro activity and beta-lactamase stability. J Antimicrob Chemother. 1985 Sep;16(3):315-25. [2]. Therapeutic efficacy of cefpiramide and cefoperazone alone and in combination with gentamicin against pseudomonal infections in neutropenic mice. Chemotherapy. 1986;32(2):166-72. [3]. The in vitro activity and beta-lactamase stability of cefpiramide compared with other beta-lactam antibiotics. Diagn Microbiol Infect Dis. 1985 Nov;3(6):479-88. |
||
| Additional Infomation |
Cefpiramide sodium is the sodium salt of cefpiramide. It contains a cefpiramide(1-). Cefpiramide Sodium is the sodium salt form of cefpiramide, a third-generation, semi-synthetic, beta-lactam cephalosporin antibiotic with antibacterial activity. Cefpiramide binds to penicillin-binding proteins (PBPs), transpeptidases that are responsible for crosslinking of peptidoglycan. By preventing crosslinking of peptidoglycan, cell wall integrity is lost and cell wall synthesis is halted. |
Solubility Data
| Solubility (In Vitro) |
DMSO : 39~100 mg/mL ( 61.45~157.57 mM ) Water : ~100 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (3.28 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (3.28 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (3.28 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.08 mg/mL (3.28 mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5757 mL | 7.8787 mL | 15.7575 mL | |
| 5 mM | 0.3151 mL | 1.5757 mL | 3.1515 mL | |
| 10 mM | 0.1576 mL | 0.7879 mL | 1.5757 mL |