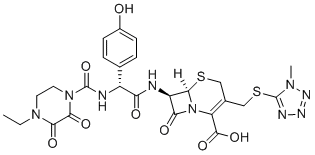
Cefoperazone (trade name Cefobid) is a third-generation, semisynthetic broad-spectrum cephalosporin antibiotic proposed to be effective against Pseudomonas infections. It inhibits rMrp2-mediated [3H]E217βG uptake with IC50 of 199 μM. It is one of few cephalosporin antibiotics effective in treating Pseudomonas bacterial infections which are otherwise resistant to these antibiotics. It was patented in 1974 and approved for medical use in 1981. Cefoperazone/sulbactam (Sulperazon) is a co-formulation with sulbactam. Cefoperazone has a broad spectrum of activity and has been used to target bacteria responsible for causing infections of the respiratory and urinary tract, skin, and the female genital tract. The following represents MIC susceptibility data for a few medically significant microorganisms. Cefoperazone exerts its bactericidal effect by inhibiting the bacterial cell wall synthesis, and sulbactam acts as a beta-lactamase inhibitor, to increase the antibacterial activity of cefoperazone against beta-lactamase-producing organisms.
Physicochemical Properties
| Molecular Formula | C25H27N9O8S2 |
| Molecular Weight | 645.6674 |
| Exact Mass | 645.142 |
| Elemental Analysis | C, 46.51; H, 4.22; N, 19.52; O, 19.82; S, 9.93 |
| CAS # | 62893-19-0 |
| Related CAS # | Cefoperazone sodium salt;62893-20-3;Cefoperazone-d5;2410425-70-4;Cefoperazone dihydrate;113826-44-1 |
| PubChem CID | 44187 |
| Appearance | White to off-white solid powder |
| Density | 1.8±0.1 g/cm3 |
| Melting Point | 169-171ºC |
| Index of Refraction | 1.819 |
| LogP | 1.43 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 9 |
| Heavy Atom Count | 44 |
| Complexity | 1250 |
| Defined Atom Stereocenter Count | 3 |
| SMILES | S1C([H])([H])C(C([H])([H])SC2=NN=NN2C([H])([H])[H])=C(C(=O)O[H])N2C([C@]([H])([C@@]12[H])N([H])C([C@@]([H])(C1C([H])=C([H])C(=C([H])C=1[H])O[H])N([H])C(N1C(C(N(C([H])([H])C([H])([H])[H])C([H])([H])C1([H])[H])=O)=O)=O)=O)=O |
| InChi Key | GCFBRXLSHGKWDP-XCGNWRKASA-N |
| InChi Code | InChI=1S/C25H27N9O8S2/c1-3-32-8-9-33(21(39)20(32)38)24(42)27-15(12-4-6-14(35)7-5-12)18(36)26-16-19(37)34-17(23(40)41)13(10-43-22(16)34)11-44-25-28-29-30-31(25)2/h4-7,15-16,22,35H,3,8-11H2,1-2H3,(H,26,36)(H,27,42)(H,40,41)/t15-,16-,22-/m1/s1 |
| Chemical Name | (6R,7R)-7-((R)-2-(4-ethyl-2,3-dioxopiperazine-1-carboxamido)-2-(4-hydroxyphenyl)acetamido)-3-(((1-methyl-1H-tetrazol-5-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| Synonyms | T1551; T-1551; T 1551; Trade name: Cefobid; Cefozon; C06883; C-06883; C 06883 D07645; D-07645; D 07645; Cefobid; Cefoperazono; Cefoperazonum; Peracef; Cefoperazone acid; Cefoperazone (Cefobid); |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | β-lactam |
| ln Vitro | Granulocytopenic mice treated with cefoperazone (aerosol treatment, final concentration of 60 μg/mL in lung homogenate) are protected against acute Pseudomonas aeruginosa pneumonia[2]. |
| ln Vivo | In the present study, we attempted to identify the membrane permeation process(es) primarily involved in the molecular-weight-dependent biliary excretion of beta-lactam antibiotics. A search of the literature indicated that the molecular weight threshold operates mainly in the transport process across bile canalicular membranes. We confirmed that biliary clearance of the model biliary-excretion-type cephalosporin cefoperazone was reduced to 10% of the control in Eisai hyperbilirubinemic rats, which are genetically deficient in multidrug resistance-associated protein (Mrp) 2, indicating that Mrp2 plays a major role as an efflux transporter on the canalicular membranes. ATP-dependent uptake of several cephalosporins including cefoperazone, cefbuperazone, cefpiramide, and ceftriaxone, all of which are mainly excreted into bile, was confirmed in membrane vesicles from Sf9 cells transfected with rat Mrp2. Both the inhibitory potency of the cephalosporins for Mrp2-mediated transport and the uptake of cephalosporins by Mrp2-expressing vesicles were molecular weight-dependent, suggesting that Mrp2 is one of the major transporters involved in molecular weight-dependent biliary excretion. An uptake study in membrane vesicles of Sf9 cells transfected with breast cancer resistance protein (Bcrp) revealed that Bcrp accepts cefoperazone, cefbuperazone, cefpiramide, cefotetan, ceftriaxone, cefotiam, cefamandole, and cefazolin as substrates, and Bcrp-mediated transport was also molecular weight-dependent, suggesting that Bcrp also contributes to molecular weight-dependent biliary excretion of beta-lactam antibiotics in rats[3]. |
| Enzyme Assay | Cefoperazone, a new semisynthetic cephalosporin, has a broad spectrum of antibacterial activity. It is as active as cefazolin and cefamandole against gram-positive bacteria and is more active than cefazolin and cefamandole against such gram-negative bacilli as Escherichia coli, Klebsiella pneumoniae, Proteus species, Pseudomonas aeruginosa, Citrobacter freundii, Enterobacter cloacae, and Serratia marcescens. The superiority of cefoperazone over cefazolin and cefamandole with respect to activity against P. aeruginosa by more than 200-fold was especially remarkable. As with other beta-lactam antibiotics, there was only a small spread between the minimum inhibitory concentrations and the minimum bactericidal concentrations of cefoperazone and a significant decrease in activity with an increase in inoculum size. Activity was not altered significantly by the addition of human serum to the test medium. Cefoperazone is relatively stable to hydrolysis to beta-lactamases produced by gram-negative bacteria. Relative rates of hydrolysis of cefoperazone by cephalosporinases are 7.0 to 0.01, with reference to cephaloridine hydrolysis (base, 100). Cefoperazone is also more stable than penicillin G and cephaloridine to various types of penicillinases[1]. |
| Animal Protocol | The pharmacokinetics of cefoperazone in normal subjects, and in patients with hepatic and renal dysfunction are reviewed. After intravenous administration of 2 g of cefoperazone, levels in serum ranged from 202 to 375 microgram/ml depending on the period of drug administration. After intramuscular injection of 2 g of cefoperazone, the mean peak serum level was 111 microgram/ml at 1.5 hours. At 12 hours after dosing, mean serum levels were still 2 to 4 microgram/ml. Cefoperazone was 90% bound to serum proteins. The apparent volume of distribution was 10 to 13L. The half-life of the drug varied from 1.6 to 2.4 hours; serum clearance was between 75 and 96 ml/min. Urinary excretion was rapid, but only 15 to 36% of the cefoperazone dose was recovered in the urine. Renal clearance ranged from 14 to 25 ml/min. Urine levels of cefoperazone in excess of 32 microgram/ml were maintained for at least 12 hours. Biliary levels of cefoperazone were many-fold higher than serum levels; peak bile concentrations from 675 to 6000 microgram/ml were obtained. Severe hepatic dysfunction was associated with a 2- to 4-fold increase in the half-life of cefoperazone. In patients with relatively complete biliary obstruction, over 90% of the dose was recovered in the urine. In contrast, the serum kinetics of cefoperazone were not significantly altered in patients with renal impairment. The human pharmacology of cefoperazone is similar to cephazolin in terms of serum concentrations, half-life, protein binding, and apparent volume of distribution, but markedly different in terms of biliary and renal excretion. Since biliary excretion is normally the primary route of cefoperazone elimination, dosage modification should only be required in the presence of severe biliary obstruction or concomitant renal and hepatic dysfunction. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Cefoperazone is excreted mainly in the bile. Metabolism / Metabolites No significant quanitity of metabolites have been identified in urine. Biological Half-Life The mean serum half-life is approximately 2.0 hours, independent of the route of administration. |
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Cefoperazone is no longer marketed in the United States. Limited information indicates cefoperazone produces low levels in milk that are not expected to cause adverse effects in breastfed infants. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush have been reported with cephalosporins, but these effects have not been adequately evaluated. Cefoperazone is acceptable in nursing mothers. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding The degree of reversible protein binding varies with the serum concentration from 93% at 25 mcg/mL to 90% at 250 mcg/mL and 82% at 500 mcg/mL. Cefotetan is 88% plasma protein bound. |
| References |
[1]. In vitro antibacterial activity of cefoperazone (T-1551), a new semisynthetic cephalosporin. Antimicrob Agents Chemother. 1979 Dec;16(6):731-5. [2]. Aerosol treatment with cefoperazone or gentamicin protects granulocytopenic mice from acute Pseudomonas aeruginosa pneumonia. European Journal of Pharmaceutical Sciences Volume 1, Issue 6, June 1994, Pages 285-289. [3]. Drug Metab Dispos, 2008. 36(6): p. 1088-96. [4]. Drugs, 1981. 22 Suppl 1: p. 35-45. |
| Additional Infomation |
Pharmacodynamics Cefoperazone is a third generation cephalosporin antibiotic. Cefoperazone exerts its bactericidal effect by inhibiting the bacterial cell wall synthesis |
Solubility Data
| Solubility (In Vitro) |
DMSO : ≥ 100 mg/mL (~154.88 mM ) H2O : ~0.1 mg/mL (~0.15 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (3.87 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (3.87 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (3.87 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 30% propylene glycol+ 5% Tween 80+ 65% D5W: 30mg/ml (46.46mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5488 mL | 7.7439 mL | 15.4878 mL | |
| 5 mM | 0.3098 mL | 1.5488 mL | 3.0976 mL | |
| 10 mM | 0.1549 mL | 0.7744 mL | 1.5488 mL |