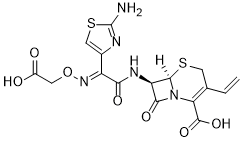
Cefixime (FR-17027; Unixime; Cefiximum; Cefixima; FK-027; CL-284635) is a novel and potent third generation cephalosporin antibiotic with activity against a variety of Gram-negative bacteria, including E. coli, K. pneumoniae, and H. influenzae. Cefixime binds to penicillin-binding proteins and is stable to many penicillinases and β-lactamases. It is an effective, orally-active cephalosporin with applications in acute otitis media, respiratory tract infections, and urinary tract infections.
Physicochemical Properties
| Molecular Formula | C16H15N5O7S2 |
| Molecular Weight | 453.45 |
| Exact Mass | 453.041 |
| Elemental Analysis | C, 42.38; H, 3.33; N, 15.44; O, 24.70; S, 14.14 |
| CAS # | 79350-37-1 |
| Related CAS # | Cefixime trihydrate;125110-14-7 |
| PubChem CID | 5362065 |
| Appearance | Off-white to slightly yellow solid powder. |
| Density | 1.9±0.1 g/cm3 |
| Melting Point | 218-225°C |
| Index of Refraction | 1.811 |
| LogP | 1 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 8 |
| Heavy Atom Count | 30 |
| Complexity | 861 |
| Defined Atom Stereocenter Count | 2 |
| SMILES | C(C1=C(C=C)CS[C@@H]2[C@@H](C(N12)=O)NC(=O)/C(/C1=CSC(N)=N1)=N\OCC(=O)O)(=O)O |
| InChi Key | OKBVVJOGVLARMR-VINNURBNSA-N |
| InChi Code | InChI=1S/C16H15N5O7S2/c1-2-6-4-29-14-10(13(25)21(14)11(6)15(26)27)19-12(24)9(20-28-3-8(22)23)7-5-30-16(17)18-7/h2,5,10,14H,1,3-4H2,(H2,17,18)(H,19,24)(H,22,23)(H,26,27)/b20-9+/t10-,14-/m1/s1 |
| Chemical Name | (6R,7R)-7-[[(2E)-2-(2-amino-1,3-thiazol-4-yl)-2-(carboxymethoxyimino)acetyl]amino]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| Synonyms | Cefixima; FR17027; Suprax; Trihydrate, Cefixime; FK 027; FK-027; FK027; FR 17027; FR-17027;Cefixim; Cefixoral; Unixime; Cefiximum; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | β-lactam |
| ln Vitro | With a MIC90 value of 0.25 μg/mL, cefixime exhibits strong antibacterial activity against clinical isolates of Salmonella typhi. It also exhibits antibacterial activity against strains that are resistant to amoxicillin (HY-B0467A) and produce β-lactamase. |
| ln Vivo | Mice challenged with FA1090 (ESC-susceptible strain) have lower bacterial burdens when cefixime (0.75–60 mg/kg) is administered orally[4]. Cefixime (50 or 150 mg/kg, oral gavage) alters the composition and diversity of the C57BL/6J mice's gut microbiota, resulting in a decrease in the microbial community's diversity and a shift toward a predominate Firmicutes phylum[5]. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion With oral administration of cefixime, about 40%-50% is absorbed whether administered with or without food. However, time to maximal absorption is increased approximately 0.8 hours when administered with food. Cefixime administered as an single oral 200 mg tablet in healthy male volunteers had a corresponding Cmax of 3.25 mg/L and a corresponding Tmax of 4 hours. Administration of cefixime as a 200 mg oral solution in healthy volunteers results in a Cmax of 3.22 micrograms/mL, while administration of 200 mg and 400 mg cefixime capsules results in a Cmax of 2.92 micrograms/mL and 4.84 micrograms/mL, respectively. Administration of cefixime as a 200 mg intravenous solution, a 200 mg oral solution, a 200 mg capsule, and 400 mg capsule results in mean areas under the curve (AUC) of 47.0 μg.h/mL, 26.0 μg.h/mL, 23.6 μg.h/mL, and 39.4 μg.h/mL, respectively. Approximately 50% of absorbed cefixime is excreted unchanged in the urine in 24 hours. Cefixime has a volume of distribution averaging 0.1 L/kg of body weight when administered orally. Cefixime administered as an oral suspension with a dose of 8 mg/kg in children with urinary tract infections aged from 6 to 13 years resulted in a mean apparent total clearance rate of 4.74 ml/min/kg. Metabolism / Metabolites There is no evidence of metabolism of cefixime _in vivo_. Biological Half-Life Cefixime has a serum half-life averaging 3 to 4 hours in healthy subjects and is independent of dosage form. It has ranged up to 9 hours in some normal volunteers. In individuals with severe renal impairment (5 to 20 mL/min creatinine clearance), the half-life of cefixime increased to an average of 11.5 hours. |
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Although no information is available on the use of cefixime during breastfeeding, cephalosporins are generally not be expected to cause adverse effects in breastfed infants. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush have been reported with cephalosporins, but these effects have not been adequately evaluated. Cefixime is acceptable in nursing mothers. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Approximately 65% of cefixime is bound to serum protein, the serum protein binding is also concentration-independent. |
| References |
[1]. In vitro activity of a new broad spectrum, beta-lactamase-stable oral cephalosporin, cefixime. Pediatr Infect Dis J. 1987 Oct;6(10):958-62. [2]. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother. 2012 Mar;56(3):1273-80. [3]. Antibacterial activity of cefixime against Salmonella typhi and applicability of Etest. J Infect Chemother. 1999 Sep;5(3):176-179. [4]. Pharmacokinetic Data Are Predictive of In Vivo Efficacy for Cefixime and Ceftriaxone against Susceptible and Resistant Neisseria gonorrhoeae Strains in the Gonorrhea Mouse Model. Antimicrob Agents Chemother. 2019 Feb 26;63(3):e01644-18. [5]. Restoration of cefixime-induced gut microbiota changes by Lactobacillus cocktails and fructooligosaccharides in a mouse model. Microbiol Res. 2017 Jul;200:14-24. |
| Additional Infomation |
Pharmacodynamics Cefixime, a broad-spectrum antibiotic, is an orally-active third-generation semisynthetic cephalosporin. Like other cephalosporins, the antibacterial action of cefixime results from inhibition of cell wall synthesis. Also like other cephalosporins, cefixime is stable when in the presence of certain beta-lactamase enzymes, which means certain organisms resistant to penicillins and some cephalosporins due to the presence of beta-lactamases could be susceptible to cefixime. Use of cefixime can result in hypersensitivity reactions including anaphylactic/anaphylactoid reactions and _Clostridium difficile_-associated diarrhea (CDAD); it may also be associated with a fall in prothrombin activity. Cefixime doses should be adjusted for patients that have renal impairment and patients undergoing continuous ambulatory peritoneal dialysis (CAPD) and hemodialysis (HD), while patients on dialysis should be monitored while taking cefixime. |
Solubility Data
| Solubility (In Vitro) |
DMSO : 90~100 mg/mL ( 198.47~220.53 mM ) Ethanol : ~1 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (5.51 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (5.51 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (5.51 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.5 mg/mL (5.51 mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2053 mL | 11.0266 mL | 22.0531 mL | |
| 5 mM | 0.4411 mL | 2.2053 mL | 4.4106 mL | |
| 10 mM | 0.2205 mL | 1.1027 mL | 2.2053 mL |