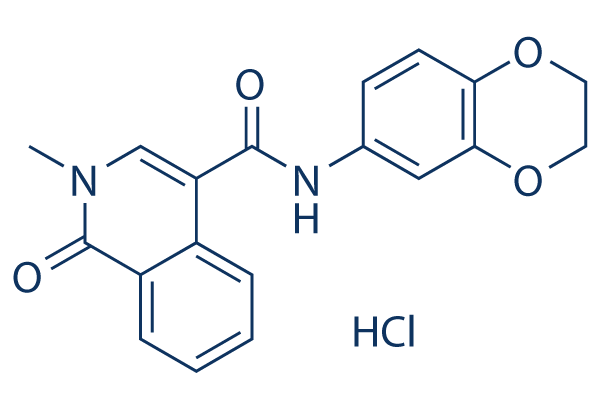
CeMMEC1, an N-methylisoquinolinone analog, is a novel and potent inhibitor of TAF4 with anticancer activity. Moreover, it inhibits the second bromodomain of TAF1 by blocking BRD4 (Kd = 1.8 µM; IC50 = 0.9 µM). CeMMEC1 only very weakly bound BRD4, but it exhibited strong affinity for the second bromodomain of TAF1, CREBBP, EP300, and BRD9. It doesn't attach to BRD4's first or second bromodomains. Bromodomain pharmacological inhibitors offer potential treatment advantages for a range of malignancies.
Physicochemical Properties
| Molecular Formula | C19H16N2O4 |
| Molecular Weight | 336.341344833374 |
| Exact Mass | 336.11 |
| Elemental Analysis | C, 67.85; H, 4.80; N, 8.33; O, 19.03 |
| CAS # | 440662-09-9 |
| Related CAS # | 440662-09-9 |
| PubChem CID | 24152379 |
| Appearance | White to off-white solid powder |
| Density | 1.4±0.1 g/cm3 |
| Boiling Point | 590.1±50.0 °C at 760 mmHg |
| Flash Point | 310.7±30.1 °C |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.673 |
| LogP | 2.69 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Heavy Atom Count | 25 |
| Complexity | 576 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | O1CCOC2C=CC(=CC1=2)NC(C1=CN(C)C(C2C=CC=CC1=2)=O)=O |
| InChi Key | PEOQAZBGLOADFJ-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C19H16N2O4/c1-21-11-15(13-4-2-3-5-14(13)19(21)23)18(22)20-12-6-7-16-17(10-12)25-9-8-24-16/h2-7,10-11H,8-9H2,1H3,(H,20,22) |
| Chemical Name | N-(2,3-dihydro-1,4-benzodioxin-6-yl)-2-methyl-1-oxoisoquinoline-4-carboxamide |
| Synonyms | Ce MMEC1; Ce-MMEC1; CeMMEC1 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | TAF1(2) ( Kd = 1.8 μM ); TAF1 ( IC50 = 0.9 μM ) | ||
| ln Vitro |
|
||
| Enzyme Assay | The EPIgeneous Binding Domain kit B is used for TAF1 binding assays. The displacement of an acetylated biotin peptide from a GST-tagged TAF1 protein using HTRF in conjunction with a streptavidin-conjugated acceptor and an Eu3+-conjugated GST antibody donor indicates binding. Using an Echo 525 Liquid Handler, compounds (CeMMEC1) are dispensed into ProxiPlate-384 Plus assay plates. 5 nM TAF1-GST, 50 nM peptide (SGRGK (ac)GGK (ac)GGAK (ac)RHRK (biotin)-acid), 6.25 nM Streptavidin-XL665, 1:200 Anti-GST-Eu3+ cryptate, and 0.1% DMSO are used in binding assays, which are carried out in a final volume of 20 μL. Utilizing a Multidrop combi, assay reagents are applied to plates and allowed to sit at room temperature for three hours. A PHERAstar microplate reader with the HTRF module and dual emission protocol is used to measure fluorescence (A = 320 nm excitation, 665 nm emission, and B = 320 nm excitation, 620 nm emission). HTRF ratio (channel A/B × 10,000) is obtained by processing raw data and is utilized in the creation of IC50 curves[1]. | ||
| Cell Assay | Target genes like the MYC oncogene are transcriptionally activated by proteins of the BET family that contain a bromodomain and can detect acetylation of histone lysine. BET domain pharmacological inhibitors offer potential treatment advantages for a range of malignancies. A high-diversity chemical compound screen was conducted to identify agents that have the ability to alter the BRD4-dependent heterochromatization of a generic reporter in human cells. We found small molecules that mimic BRD4 inhibition without direct engagement in addition to new and known compounds that target BRD4. One such substance was a strong inhibitor of TAF1's second bromodomain. By using this inhibitor, we were able to ascertain that TAF1 functions in concert with BRD4 to regulate the growth of cancer cells, rendering TAF1 a desirable epigenetic target in MYC-driven cancers. | ||
| Animal Protocol |
|
||
| References |
[1]. Mapping the chemical chromatin reactivation landscape identifies BRD4-TAF1 cross-talk. Nat Chem Biol. 2016 Jul;12(7):504-10. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (7.43 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9732 mL | 14.8659 mL | 29.7318 mL | |
| 5 mM | 0.5946 mL | 2.9732 mL | 5.9464 mL | |
| 10 mM | 0.2973 mL | 1.4866 mL | 2.9732 mL |