Physicochemical Properties
| Molecular Formula | C16H12O5 |
| Molecular Weight | 284.2635 |
| Exact Mass | 284.068 |
| Elemental Analysis | C, 67.60; H, 4.26; O, 28.14 |
| CAS # | 20575-57-9 |
| Related CAS # | 20575-57-9 |
| PubChem CID | 5280448 |
| Appearance | White to off-white solid powder |
| Density | 1.4±0.1 g/cm3 |
| Boiling Point | 536.8±50.0 °C at 760 mmHg |
| Flash Point | 205.7±23.6 °C |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.669 |
| LogP | 2.41 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Heavy Atom Count | 21 |
| Complexity | 432 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | O1C([H])=C(C(C2C([H])=C([H])C(=C([H])C1=2)O[H])=O)C1C([H])=C([H])C(=C(C=1[H])O[H])OC([H])([H])[H] |
| InChi Key | ZZAJQOPSWWVMBI-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C16H12O5/c1-20-14-5-2-9(6-13(14)18)12-8-21-15-7-10(17)3-4-11(15)16(12)19/h2-8,17-18H,1H3 |
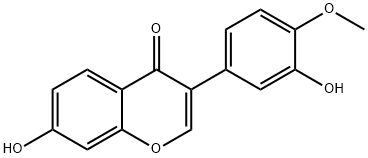
| Chemical Name | 7-hydroxy-3-(3-hydroxy-4-methoxyphenyl)chromen-4-one |
| Synonyms | Calycosin |
| HS Tariff Code | 2934.99.03.00 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ADME/Pharmacokinetics |
Metabolism / Metabolites Calycosin and calycosin-7-O-beta-d-glucoside are two main bioactive isoflavonoids in Astragali Radix. To profile the metabolites of calycosin in rat hepatic 9000 g supernatant incubation system and the metabolites of calycosin-7-O-beta-d-glucoside in rat urine, high performance liquid chromatography with diode array detector and combined with electrospray ionization ion trap time-of-flight multistage mass spectrometry (HPLC-DAD-ESI-IT-TOF-MSn) technique was used. Totally, 24 new in vitro metabolites of calycosin and 33 new in vivo metabolites of calycosin-7-O-beta-d-glucoside were identified. Monoglucosylation, monopentosylation, demethylation, dehydroxylation, dimerization, and trimerization were found to be new in vitro metabolic reactions of calycosin; hydroxylation and hydrogenation were new metabolic reactions of calycosin-7-O-beta-d-glucoside in vivo. The major metabolic reactions of calycosin in rat hepatic 9000 g supernatant incubation system were monohydroxylation on A-ring, dimerization (CO coupling), dimerization (CC coupling) and dehydroxylation; the major phase I metabolic reactions of calycosin-7-O-beta-d-glucoside in rats were deglycosylation, hydroxylation, demethylation and dehydroxylation. Hydroxylation, dehydroxylation, and demethylation were common metabolic pathways to calycosin and calycosin-7-O-beta-d-glucoside, and some of their metabolites formed through these reactions, such as 8-hydroxycalycosin (S10, M10), pratensein (5-hydroxycalycosin, S19, M27) and formononetin (S22, M28), daidzein (M22), 7,3',4'-trihydroxyisoflavone (S13, aglycon of M3 and M8), equol (aglycon of M19 and M20) had been reported to have many bioactivities related to the pharmacological effects of calycosin and calycosin-7-O-beta-d-glucoside. These findings would enhance understanding of the metabolism and real active forms of calycosin and calycosin-7-O-beta-d-glucoside. In vivo and in vitro metabolites of calycosin-7-O-beta-D-glucopyranoside in rats were identified using a specific and sensitive high performance liquid chromatography-tandem mass spectrometry (HPLC-MS(n)) method. The parent compound and twelve metabolites were found in rat urine after oral administration of calycosin-7-O-beta-D-glucopyranoside. The parent compound and six metabolites were detected in rat plasma. In heart, liver, spleen, lung and kidney samples, respectively, six, eight, seven, nine and nine metabolites were identified, in addition to the parent compound. Three metabolites, but no trace of parent drug, were found in the rat intestinal flora incubation mixture and feces, which demonstrated cleavage of the glycosidic bond of the parent compound in intestines. The main phase I metabolic pathways of calycosin-7-O-beta-D-glucopyranoside in rats were deglycosylation, dehydroxylation and demethylation reactions; phase II metabolism included sulfation, methylation, glucuronidation and glycosylation (probably). Furthermore, two metabolites commonly found in rat urine, plasma and tissues were isolated from feces and characterized by NMR. The antiviral activities of the metabolite calycosin against coxsackie virus B3 (CVB3) and human immunodeficiency virus (HIV) were remarkably stronger than those of calycosin-7-O-beta-D-glucopyranoside. |
| Toxicity/Toxicokinetics |
Interactions Danggui Buxue Tang (DBT), a herbal decoction contains Astragali Radix (AR) and Angelicae Sinensis Radix (ASR), has been used as a health food supplement in treating menopausal irregularity in women for more than 800 years in China. Several lines of evidence indicate that the synergistic actions of AR and ASR in this herbal decoction leading to a better pharmacological effect of DBT. Here, the role of different herbs in directing the transport of active ingredients of DBT was determined. A validated RRLC-QQQ-MS/MS method was applied to determinate the permeability of ingredients across the Caco-2 cell monolayer. AR-derived chemicals, including astragaloside IV, calycosin and formononetin, as well as ASR-derived chemicals, including ferulic acid and ligustilide, were determined by RRLC-QQQ-MS/MS. The pharmacokinetic results showed that the membrane permeabilities of calycosin and formononetin, two of the major flavonoids in AR, could be markedly increased in the presence of ASR extract: this induction effect could be mediated by ferulic acid deriving from ASR. In contrast, the extract of AR showed no effect on the chemical permeability. The current results suggested that the ingredients of ASR (such as ferulic acid) could enhance the membrane permeability of AR-derived formononetin and calycosin in cultured Caco-2 cells. The possibility of herb-drug synergy within DBT was proposed here. |
| References |
[1]. Calycosin induces apoptosis in human ovarian cancer SKOV3 cells by activating caspases and Bcl-2 family proteins. Tumour Biol. 2015 Feb 12. [2]. Calycosin and genistein induce apoptosis by inactivation of HOTAIR/p-Akt signaling pathway in human breast cancer MCF-7 cells. Cell Physiol Biochem. 2015;35(2):722-8. [3].Calycosin suppresses breast cancer cell growth via ERβ-dependent regulation of IGF-1R, p38 MAPK and PI3K/Akt pathways. PLoS One. 2014 Mar 11;9(3):e91245. [4]. Calycosin promotes proliferation of estrogen receptor-positive cells via estrogen receptors and ERK1/2 activation in vitro and in vivo. Cancer Lett. 2011 Sep 28;308(2):144-51. |
| Additional Infomation |
Calycosin is a member of the class of 7-hydroxyisoflavones that is 7-hydroxyisoflavone which is substituted by an additional hydroxy group at the 3' position and a methoxy group at the 4' position. It has a role as a metabolite and an antioxidant. It is a member of 7-hydroxyisoflavones and a member of 4'-methoxyisoflavones. It is functionally related to an isoflavone. It is a conjugate acid of a calycosin(1-). Calycosin has been reported in Bowdichia virgilioides, Glycyrrhiza pallidiflora, and other organisms with data available. Mechanism of Action ... The present study was designed to explore the therapeutic effect of calycosin, an active component from A. radix, on AGEs-induced macrophages infiltration in HUVECs. ...Transwell HUVEC-macrophage co-culture system was established to evaluate macrophage migration and adhesion. Immunocytochemistry was applied to examine TGF-beta1, ICAM-1 and RAGE protein expressions; real-time PCR was carried out to determine mRNA expression of TGF-beta1, ICAM-1 and RAGE. Immunofluorescence was carried out to observe estrogen receptor-alpha, ICAM-1, RAGE expression and the phosphorylation status of ERK1/2 and NF-kappaB. Calycosin significantly reduced AGEs-induced macrophage migration and adhesion to HUVEC. Pre-treatment with calycosin strikingly down-regulated HUVEC TGF-beta1, ICAM-1 and RAGE expressions in both protein and mRNA levels. Furthermore, calycosin incubation significantly increased estrogen receptor expression and reversed AGEs-induced ERK1/2 and NF-kappaB phosphorylation and nuclear translocation in HUVEC, and this effect of calycosin could be inhibited by estrogen receptor inhibitor, ICI182780. These findings suggest that calycosin can reduce AGEs-induced macrophage migration and adhesion to endothelial cells and relieve the local inflammation; furthermore, this effect was via estrogen receptor-ERK1/2-NF-kappaB pathway. |
Solubility Data
| Solubility (In Vitro) | DMSO: ≥ 100 mg/mL (~351.8 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (8.79 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (8.79 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (8.79 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.5179 mL | 17.5895 mL | 35.1791 mL | |
| 5 mM | 0.7036 mL | 3.5179 mL | 7.0358 mL | |
| 10 mM | 0.3518 mL | 1.7590 mL | 3.5179 mL |