CX-5461 (CX 5461; CX5461) is a novel, selective and orally bioavailable inhibitor of rRNA synthesis and rDNA transcription inhibitor with potential antitumor activity. It inhibits rRNA transcription driven by Pol I in a variety of cell types, including MIA PaCa-2, A375, and HCT-116 cells, with an IC50 of 142 nM. CX-5461 exhibits strong in vivo antitumor efficacy against solid human tumors in models of murine xenograft.
Physicochemical Properties
| Molecular Formula | C27H27N7O2S | |
| Molecular Weight | 513.61 | |
| Exact Mass | 513.194 | |
| Elemental Analysis | C, 63.14; H, 5.30; N, 19.09; O, 6.23; S, 6.24 | |
| CAS # | 1138549-36-6 | |
| Related CAS # | 1138549-36-6; 2101314-20-7 (HCl) | |
| PubChem CID | 25257557 | |
| Appearance | White solid powder | |
| Density | 1.5±0.1 g/cm3 | |
| Boiling Point | 739.9±60.0 °C at 760 mmHg | |
| Flash Point | 401.3±32.9 °C | |
| Vapour Pressure | 0.0±2.4 mmHg at 25°C | |
| Index of Refraction | 1.740 | |
| LogP | -0.81 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 9 | |
| Rotatable Bond Count | 4 | |
| Heavy Atom Count | 37 | |
| Complexity | 915 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | S1C2=C([H])C([H])=C([H])C([H])=C2N2C1=C(C(N([H])C([H])([H])C1C([H])=NC(C([H])([H])[H])=C([H])N=1)=O)C(C1C([H])=C([H])C(=NC2=1)N1C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])C([H])([H])C1([H])[H])=O |
|
| InChi Key | XGPBJCHFROADCK-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C27H27N7O2S/c1-17-14-29-18(15-28-17)16-30-26(36)23-24(35)19-8-9-22(33-11-5-10-32(2)12-13-33)31-25(19)34-20-6-3-4-7-21(20)37-27(23)34/h3-4,6-9,14-15H,5,10-13,16H2,1-2H3,(H,30,36) | |
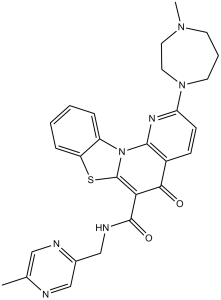
| Chemical Name | 2-(4-methyl-1,4-diazepan-1-yl)-N-[(5-methylpyrazin-2-yl)methyl]-5-oxo-[1,3]benzothiazolo[3,2-a][1,8]naphthyridine-6-carboxamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | rRNA synthesis, MIA PaCa-2 cells ( IC50 = 54 nM ); rRNA synthesis, A375 cells ( IC50 = 113 nM ); rRNA synthesis, HCT-116 cells ( IC50 = 142 nM ) | |
| ln Vitro |
|
|
| ln Vivo |
|
|
| Enzyme Assay | CX-5461-related effects on transcription are measured by qRT-PCR, which quantifies two short-lived RNA transcripts (half-lives ~20-30 minutes), one produced by Pol I and another by Pol II. As the Pol I transcript, the 45S pre-rRNA functioned, while the comparator Pol II transcript was the protooncogene c-myc mRNA. Cell stress in general is known to impact both Pol I and Pol II transcription. Cells are only exposed to test agents for a brief amount of time (2 hours) in order to reduce any possible effects of such stress. There is enough time for CX-5461 to impact the synthesis of these transcripts, resulting in a reduction of more than 90%. | |
| Cell Assay | The following day, cells are treated with CX-5461 dose response for 96 hours after being plated on 96-well plates. Alamar Blue and CyQUANT assays are used to measure cell viability. | |
| Animal Protocol |
|
|
| References |
[1]. Targeting RNA polymerase I with an oral small molecule CX-5461 inhibits ribosomal RNA synthesis and solid tumor growth. Cancer Res. 2011 Feb 15;71(4):1418-30. [2]. Inhibition of RNA Polymerase I as a Therapeutic Strategy to Promote Cancer-Specific Activation of p53. Cancer Cell. 2012 Jul 10;22(1):51-65. |
|
| Additional Infomation |
CX-5461 is an organic heterotetracyclic compound that is 5-oxo-5H-[1,3]benzothiazolo[3,2-a][1,8]naphthyridine-6-carboxylic acid substituted by a 4-methyl-1,4-diazepan-1-yl group at position 2 and in which the carboxy group at position 6 is substituted by a [(5-methylpyrazin-2-yl)methyl]nitrilo group. An inhibitor of ribosomal RNA (rRNA) synthesis which specifically inhibits RNA polymerase I-driven transcription of rRNA in several cancer cell lines. It is currently in phase I/II clinical trials for advanced hematologic malignancies and triple-negative breast cancer with BRCA1/2 mutation. It has a role as an antineoplastic agent, an apoptosis inducer and an EC 2.7.7.6 (RNA polymerase) inhibitor. It is a diazepine, an organic heterotetracyclic compound, a member of pyrazines, a secondary carboxamide and a naphthyridine derivative. Pidnarulex is an orally bioavailable inhibitor of RNA polymerase I (Pol I), with potential antineoplastic activity. Upon oral administration, pidnarulex selectively binds to and inhibits Pol I, prevents Pol I-mediated ribosomal RNA (rRNA) synthesis, induces apoptosis, and inhibits tumor cell growth. Pol I, the multiprotein complex that synthesizes rRNA, is upregulated in cancer cells and plays a key role in cell proliferation and survival. Hyperactivated rRNA transcription is associated with uncontrolled cancer cell proliferation. Deregulated ribosomal RNA synthesis is associated with uncontrolled cancer cell proliferation. RNA polymerase (Pol) I, the multiprotein complex that synthesizes rRNA, is activated widely in cancer. Thus, selective inhibitors of Pol I may offer a general therapeutic strategy to block cancer cell proliferation. Coupling medicinal chemistry efforts to tandem cell- and molecular-based screening led to the design of CX-5461, a potent small-molecule inhibitor of rRNA synthesis in cancer cells. CX-5461 selectively inhibits Pol I-driven transcription relative to Pol II-driven transcription, DNA replication, and protein translation. Molecular studies demonstrate that CX-5461 inhibits the initiation stage of rRNA synthesis and induces both senescence and autophagy, but not apoptosis, through a p53-independent process in solid tumor cell lines. CX-5461 is orally bioavailable and demonstrates in vivo antitumor activity against human solid tumors in murine xenograft models. Our findings position CX-5461 for investigational clinical trials as a potent, selective, and orally administered agent for cancer treatment.[1] Increased transcription of ribosomal RNA genes (rDNA) by RNA Polymerase I is a common feature of human cancer, but whether it is required for the malignant phenotype remains unclear. We show that rDNA transcription can be therapeutically targeted with the small molecule CX-5461 to selectively kill B-lymphoma cells in vivo while maintaining a viable wild-type B cell population. The therapeutic effect is a consequence of nucleolar disruption and activation of p53-dependent apoptotic signaling. Human leukemia and lymphoma cell lines also show high sensitivity to inhibition of rDNA transcription that is dependent on p53 mutational status. These results identify selective inhibition of rDNA transcription as a therapeutic strategy for the cancer specific activation of p53 and treatment of hematologic malignancies.[2] RNA polymerase I (Pol I)-mediated transcription of the ribosomal RNA genes (rDNA) is confined to the nucleolus and is a rate-limiting step for cell growth and proliferation. Inhibition of Pol I by CX-5461 can selectively induce p53-mediated apoptosis of tumour cells in vivo. Currently, CX-5461 is in clinical trial for patients with advanced haematological malignancies (Peter Mac, Melbourne). Here we demonstrate that CX-5461 also induces p53-independent cell cycle checkpoints mediated by ATM/ATR signaling in the absence of DNA damage. Further, our data demonstrate that the combination of drugs targeting ATM/ATR signaling and CX-5461 leads to enhanced therapeutic benefit in treating p53-null tumours in vivo, which are normally refractory to each drug alone. Mechanistically, we show that CX-5461 induces an unusual chromatin structure in which transcriptionally competent relaxed rDNA repeats are devoid of transcribing Pol I leading to activation of ATM signaling within the nucleoli. Thus, we propose that acute inhibition of Pol transcription initiation by CX-5461 induces a novel nucleolar stress response that can be targeted to improve therapeutic efficacy. Oncotarget. 2016 Aug 2;7(31):49800-49818. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9470 mL | 9.7350 mL | 19.4700 mL | |
| 5 mM | 0.3894 mL | 1.9470 mL | 3.8940 mL | |
| 10 mM | 0.1947 mL | 0.9735 mL | 1.9470 mL |