Physicochemical Properties
| Molecular Formula | C28H29NO8 |
| Molecular Weight | 507.5318 |
| Exact Mass | 507.189 |
| CAS # | 1352914-52-3 |
| PubChem CID | 56946209 |
| Appearance | White to off-white solid powder |
| LogP | 2.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 7 |
| Heavy Atom Count | 37 |
| Complexity | 799 |
| Defined Atom Stereocenter Count | 5 |
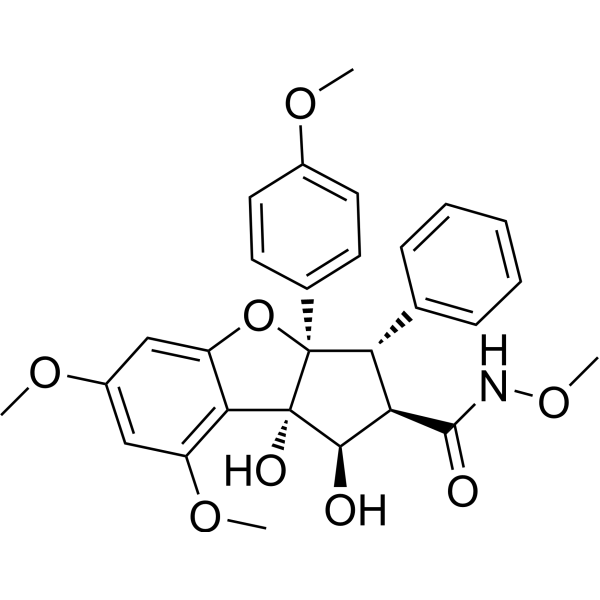
| SMILES | O1C2C([H])=C(C([H])=C(C=2[C@@]2([C@@]([H])([C@]([H])(C(N([H])OC([H])([H])[H])=O)[C@@]([H])(C3C([H])=C([H])C([H])=C([H])C=3[H])[C@]12C1C([H])=C([H])C(=C([H])C=1[H])OC([H])([H])[H])O[H])O[H])OC([H])([H])[H])OC([H])([H])[H] |
| InChi Key | KLSIFOJPWJBRFH-GWNOIRNCSA-N |
| InChi Code | InChI=1S/C28H29NO8/c1-33-18-12-10-17(11-13-18)28-23(16-8-6-5-7-9-16)22(26(31)29-36-4)25(30)27(28,32)24-20(35-3)14-19(34-2)15-21(24)37-28/h5-15,22-23,25,30,32H,1-4H3,(H,29,31)/t22-,23-,25-,27+,28+/m1/s1 |
| Chemical Name | (1R,2R,3S,3aR,8bS)-1,8b-dihydroxy-N,6,8-trimethoxy-3a-(4-methoxyphenyl)-3-phenyl-2,3-dihydro-1H-cyclopenta[b][1]benzofuran-2-carboxamide |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | eIF4 |
| ln Vitro | In MCF-10A cells (stimulated by EGF), CR-1-31-B (100 nM; 24 hours) suppresses MUC1-C translation [1]. MUC1-C abundance is decreased in MDA-MB-468 breast cancer cells by CR-1-31-B (10 and 100 nM) [1]. By translating down c-FLIP, CR-1-31B makes gallbladder cancer cells more susceptible to TRAIL-mediated apoptosis [2]. When treated with synthetic Rocaglate CR-1-31-B (24–72 hours), neuroblastoma (NB) cell lines show decreased viability, increased apoptosis rate, and changes in cell cycle distribution. A translational barrier is created when eIF4A and eIF4F are clamped onto mRNA [4]. In pancreatic cancer cells, treatment with CR-1-31-B (100 nM; 5 hours) enhances glutamine countermetabolism [5]. |
| ln Vivo | CR-1-31-B (2 mg/kg in 60 μL olive oil; i.p.; every 2 days for 28 days) in a BALB/c nude mouse model of gallbladder cancer cells Apoptosis of cells (GBC) inhibits growth and starts TRAIL induction[2]. Protein synthesis and pancreatic tumor growth are effectively inhibited by CR-1-31-B (0.2 mg/kg; intraperitoneal injection; once daily for 7 days; pancreatic ductal adenocarcinoma mouse orthotopic transplantation model) [5]. |
| Cell Assay |
Western Blot Analysis[1] Cell Types: MCF-10A cells (EGF-stimulated) Tested Concentrations: 100 nM Incubation Duration: 24 hrs (hours) Experimental Results: Blocked increases in MUC1-C abundance. Cell Viability Assay[4] Cell Types: SH-SY5Y cells and Kelly cells Tested Concentrations: 0.1-100 nM Incubation Duration: 24-72 hrs (hours) Experimental Results: A significant decrease in SH -SY5Y viability was observed at 10 nM for all time points. Dramatically diminished the viability of Kelly cells at 5 nM. The calculated IC50 at 48 h was 20 nM for SH-SY5Y and 4 nM for Kelly cells. Apoptosis Analysis[4] Cell Types: SH-SY5Y and Kelly cells Tested Concentrations: SH-SY5Y cells were treated with 10, 20, and 50 nM and Kelly cells with 1, 5, and 10 nM Incubation Duration: 24-72 hrs (hours) Experimental Results: Triggered apoptosis. |
| References |
[1]. Jin C, Rajabi H, Rodrigo CM, Porco JA Jr, Kufe D. Targeting the eIF4A RNA helicase blocks translation of the MUC1-C oncoprotein. Oncogene. 2013;32(17):2179-2188. [2]. Targeting eIF4A using rocaglate CR 1 31B sensitizes gallbladder cancer cells to TRAIL mediated apoptosis through the translational downregulation of c FLIP. Oncol Rep. 2021;45(1):230-238. [3]. Rocaglates as dual-targeting agents for experimental cerebral malaria. Proc Natl Acad Sci U S A. 2018;115(10):E2366-E2375. [4]. Eukaryotic Translation Initiation Factor 4AI: A Potential Novel Target in Neuroblastoma. Cells. 2021;10(2):301. Published 2021 Feb 2. [5]. eIF4A supports an oncogenic translation program in pancreatic ductal adenocarcinoma. Nat Commun. 2019;10(1):5151. Published 2019 Nov 13. |
Solubility Data
| Solubility (In Vitro) | DMSO : 230 mg/mL (453.18 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 5.75 mg/mL (11.33 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 57.5 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 5.75 mg/mL (11.33 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 57.5 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 5.75 mg/mL (11.33 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 57.5 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9703 mL | 9.8516 mL | 19.7033 mL | |
| 5 mM | 0.3941 mL | 1.9703 mL | 3.9407 mL | |
| 10 mM | 0.1970 mL | 0.9852 mL | 1.9703 mL |