COH29 is an orally bioavailable, aromatically substituted thiazole and inhibitor of the human ribonucleotide reductase (RNR), with potential antineoplastic activity. Upon orally bioavailable administration, the RNR inhibitor COH29 binds to the ligand-binding pocket of the RNR M2 subunit (hRRM2) near the C-terminal tail. The RNR inhibitor COH29 binds to the ligand-binding pocket of the RNR M2 subunit (hRRM2) close to the C-terminal tail after being administered orally in a bioavailable form. This prevents the hRRM1 and hRRM2 subunits from interacting with one another and obstructs the formation of the active hRRM1/hRRM2 complex of RNR. The quantity of deoxyribonucleotide triphosphates available for DNA synthesis is reduced when RNR activity is inhibited. Cell cycle arrest and development inhibition are the results of the subsequent reduction in DNA synthesis. Furthermore, this substance may inhibit the nuclear enzyme poly (ADP-ribose) polymerase (PARP) 1, which results in an accumulation of single and double strand breaks in DNA and the induction of apoptosis in addition to impeding DNA repair.
Physicochemical Properties
| Molecular Formula | C22H16N2O5S |
| Molecular Weight | 420.439 |
| Exact Mass | 420.078 |
| Elemental Analysis | C, 62.85; H, 3.84; N, 6.66; O, 19.03; S, 7.63 |
| CAS # | 1190932-38-7 |
| Related CAS # | 1190932-38-7 |
| PubChem CID | 44253415 |
| Appearance | Light yellow to yellow solid powder |
| LogP | 4.624 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Heavy Atom Count | 30 |
| Complexity | 590 |
| Defined Atom Stereocenter Count | 0 |
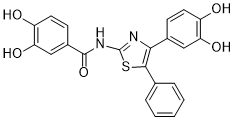
| SMILES | S1C(N([H])C(C2C([H])=C([H])C(=C(C=2[H])O[H])O[H])=O)=NC(C2C([H])=C([H])C(=C(C=2[H])O[H])O[H])=C1C1C([H])=C([H])C([H])=C([H])C=1[H] |
| InChi Key | LGGDLPSXAGQFSG-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C22H16N2O5S/c25-15-8-6-13(10-17(15)27)19-20(12-4-2-1-3-5-12)30-22(23-19)24-21(29)14-7-9-16(26)18(28)11-14/h1-11,25-28H,(H,23,24,29) |
| Chemical Name | N-[4-(3,4-dihydroxyphenyl)-5-phenyl-1,3-thiazol-2-yl]-3,4-dihydroxybenzamide |
| Synonyms | COH29; COH-29; COH 29 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | RNR ( IC50 = 8 μM ); RNR ( IC90 = 31.57±3.35 μM ) |
| ln Vitro | COH29, an RNR inhibitor, counteracts the effects of gemcitabine and hydroxyurea. It has little effect on normal fibroblasts or endothelial cells, but it significantly reduces intermittent growth in most cell lines in the NCI 60 human cancer panel, most notably advanced malignancies and lymphocytes. The ligand binding pocket of COH29-binding defense was confirmed by site-directed mutagenesis, NMR, and surface barrier biosensor investigations, which also offered proof for the assembly of the RRM1-RRM2 quaternary structure [1]. |
| ln Vivo | On day 12 of therapy, COH29 was found to cause a dose-dependent suppression of the growth of MOLT-4 xenograft tumors at twice daily tumor doses of 50 mg/kg and 100 mg/kg. Similarly, dose-inhibitory factors of xenograft tumor growth were obtained by treating TOV11D-labeled xenograft tumor models with 200, 300, or 400 mg/kg/day COH29 for seven days. Factors considerably decreased tumor growth in comparison to the control group [1]. |
| Cell Assay | Depending on the rate of growth of the cell line, 2000–5000 cells are seeded into each well of 96-well plates using 100 µL of complete medium. Following an overnight incubation period, 50 µL of culture medium containing varying concentrations of the test compound is added to each well. Following a 96-hour incubation period at 37°C, each well is supplemented with 10 mg/mL of fluorescein diacetate and 0.1% (w/v) of eosin Y. The cells are then incubated for a further 20 minutes at 37°C. Digital Imaging Microscopy System detection is used to evaluate cytotoxicity. MTS [(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)] is used to evaluate viability, as previously mentioned[1]. |
| Animal Protocol | NSG mice, female, 8–10 week old, NOD/SCID/IL2Rgamma null. The tumor volume (0.5×l×w2) of each mouse is tracked after subcutaneous injection of 5×106Molt-4 or TOV-112D cells in the right flank. COH29 in 30% solutol is given through gavage once or twice a day in doses once the tumors get to be about 70 mm3. After transplanting cancer cells, mice are killed on day 28[1]. |
| References |
[1]. A small-molecule blocking ribonucleotide reductase holoenzyme formation inhibits cancer cell growth and overcomes drug resistance.Cancer Res. 2013 Nov 1;73(21):6484-93. [2]. The Novel Ribonucleotide Reductase Inhibitor COH29 Inhibits DNA Repair In Vitro. Mol Pharmacol. 2015 Jun;87(6):996-1005. |
| Additional Infomation | RNR Inhibitor COH29 is an orally available, aromatically substituted thiazole and inhibitor of the human ribonucleotide reductase (RNR), with potential antineoplastic activity. Upon oral administration, the RNR inhibitor COH29 binds to the ligand-binding pocket of the RNR M2 subunit (hRRM2) near the C-terminal tail. This blocks the interaction between the hRRM1 and hRRM2 subunits and interferes with the assembly of the active hRRM1/hRRM2 complex of RNR. Inhibition of RNR activity decreases the pool of deoxyribonucleotide triphosphates available for DNA synthesis. The resulting decrease in DNA synthesis causes cell cycle arrest and growth inhibition. In addition, this agent may inhibit the nuclear enzyme poly (ADP-ribose) polymerase (PARP) 1, which prevents the repair of damaged DNA, and causes both the accumulation of single and double strand DNA breaks and the induction of apoptosis. RNR, an enzyme that catalyzes the conversion of ribonucleoside diphosphate to deoxyribonucleoside diphosphate, is essential for de novo DNA synthesis and plays an important role in cell growth; it is overexpressed in many cancer cell types and is associated with increased drug resistance, cancer cell growth and metastasis. |
Solubility Data
| Solubility (In Vitro) |
DMSO: 40~50 mg/mL (95.1~118.9 mM) Ethanol: 10 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (5.95 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (5.95 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (5.95 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 5 mg/mL (11.89 mM) in 30% Polyethylene glycol 12-hydroxystearate in Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3785 mL | 11.8923 mL | 23.7846 mL | |
| 5 mM | 0.4757 mL | 2.3785 mL | 4.7569 mL | |
| 10 mM | 0.2378 mL | 1.1892 mL | 2.3785 mL |