Physicochemical Properties
| Molecular Formula | C17H19F2N5O2S |
| Molecular Weight | 395.43 |
| Exact Mass | 395.122 |
| CAS # | 264233-05-8 |
| PubChem CID | 9581011 |
| Appearance | Off-white to light yellow solid powder |
| Density | 1.3±0.1 g/cm3 |
| Boiling Point | 493.6±55.0 °C at 760 mmHg |
| Flash Point | 252.3±31.5 °C |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.591 |
| LogP | 3.05 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 27 |
| Complexity | 572 |
| Defined Atom Stereocenter Count | 0 |
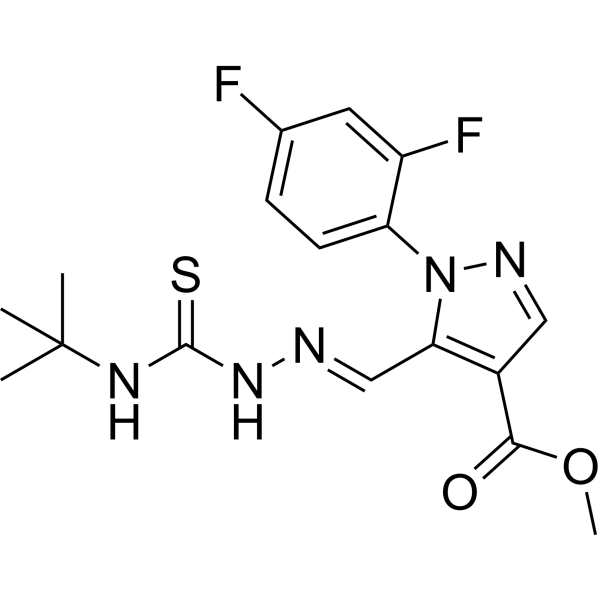
| SMILES | CC(C)(C)NC(=S)N/N=C/C1=C(C=NN1C2=C(C=C(C=C2)F)F)C(=O)OC |
| InChi Key | CYNLZIBKERMMOA-AWQFTUOYSA-N |
| InChi Code | InChI=1S/C17H19F2N5O2S/c1-17(2,3)22-16(27)23-20-9-14-11(15(25)26-4)8-21-24(14)13-6-5-10(18)7-12(13)19/h5-9H,1-4H3,(H2,22,23,27)/b20-9+ |
| Chemical Name | methyl 5-[(E)-(tert-butylcarbamothioylhydrazinylidene)methyl]-1-(2,4-difluorophenyl)pyrazole-4-carboxylate |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | The CID 2745687 (CID2745687) Ki for ERK1/2 phosphorylation using 1 μM Pamoic acid as the agonist is 18 nM[1]. In β-arrestin-2 interaction experiments, CID 2745687 (CID-2745687) is a strong antagonist limited to human GPR35 [2]. With an agonist concentration of 20 μM Zaprinast and the BRET-based GPR35-β-arrestin-2 interaction test, CID 2745687 exhibited moderate potency and concentration dependence at human GPR35, with a pIC50 of 6.70±0.09[2]. CID 2745687 (pIC50=6.27±0.08) completely counteracts Cromolyn disodium's agonistic effects [2]. |
| ln Vivo | A particular GPR35 antagonist, CID 2745687 (CID2745687; 1 mg/kg; taken orally daily for the last 4 weeks), counteracts the anti-fibrotic effects of lodoxamide[3]. |
| Animal Protocol |
Animal/Disease Models: Sixweeks old male C57BL/6 mice[3] Doses: 1 mg/kg Route of Administration: Oral administration, every day for 4 weeks Experimental Results: Inhibited Lodoxamide-mediated protective effects. |
| References |
[1]. Targeting of the orphan receptor GPR35 by pamoic acid: a potent activator of extracellular signal-regulated kinase and β-arrestin2 with antinociceptive activity. Mol Pharmacol. 2010 Oct;78(4):560-8. [2]. Antagonists of GPR35 display high species ortholog selectivity and varying modes of action. J Pharmacol Exp Ther. 2012 Dec;343(3):683-95. [3]. Lodoxamide Attenuates Hepatic Fibrosis in Mice: Involvement of GPR35. Biomol Ther (Seoul). 2019 Jun 13;28(1):92-97. |
| Additional Infomation | 5-[[[(tert-butylamino)-sulfanylidenemethyl]hydrazinylidene]methyl]-1-(2,4-difluorophenyl)-4-pyrazolecarboxylic acid methyl ester is a ring assembly and a member of pyrazoles. |
Solubility Data
| Solubility (In Vitro) | DMSO: 125 mg/mL (316.11 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (5.26 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5289 mL | 12.6445 mL | 25.2889 mL | |
| 5 mM | 0.5058 mL | 2.5289 mL | 5.0578 mL | |
| 10 mM | 0.2529 mL | 1.2644 mL | 2.5289 mL |