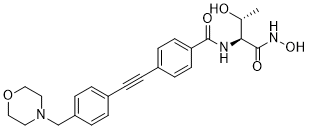
CHIR-090 (CHIR090), a novel N-aroyl-l-threonine hydroxamic acid and antibiotic, is a highly potent, slow, and tight-binding inhibitor of the LpxC deacetylase from the hyperthermophile Aquifex aeolicus. LpxC is a deacetylase involved in the biosynthesis of LPS lipid A. CHIR-090 has excellent antibiotic activity against Pseudomonas aeruginosa and Escherichia coli, as judged by disk diffusion assays. CHIR-090 is also a two-step slow, tight-binding inhibitor of E. coli LpxC with Ki = 4.0 nM, Ki* = 0.5 nM, k5 = 1.9 min-1, and k6 = 0.18 min-1. CHIR-090 at low nanomolar levels inhibits LpxC orthologues from diverse Gram-negative pathogens, including P. aeruginosa, Neisseria meningitidis, and Helicobacter pylori. In contrast, CHIR-090 is a relatively weak competitive and conventional inhibitor (lacking slow, tight-binding kinetics) of LpxC from Rhizobium leguminosarum (Ki = 340 nM), a Gram-negative plant endosymbiont that is resistant to this compound.
Physicochemical Properties
| Molecular Formula | C24H27N3O5 | |
| Molecular Weight | 437.49 | |
| Exact Mass | 437.195 | |
| Elemental Analysis | C, 65.89; H, 6.22; N, 9.60; O, 18.28 | |
| CAS # | 728865-23-4 | |
| Related CAS # |
|
|
| PubChem CID | 11546620 | |
| Appearance | White to off-white solid powder | |
| Density | 1.3±0.1 g/cm3 | |
| Index of Refraction | 1.650 | |
| LogP | 1.23 | |
| Hydrogen Bond Donor Count | 4 | |
| Hydrogen Bond Acceptor Count | 6 | |
| Rotatable Bond Count | 8 | |
| Heavy Atom Count | 32 | |
| Complexity | 680 | |
| Defined Atom Stereocenter Count | 2 | |
| SMILES | O1C([H])([H])C([H])([H])N(C([H])([H])C2C([H])=C([H])C(C#CC3C([H])=C([H])C(=C([H])C=3[H])C(N([H])[C@]([H])(C(N([H])O[H])=O)[C@@]([H])(C([H])([H])[H])O[H])=O)=C([H])C=2[H])C([H])([H])C1([H])[H] |
|
| InChi Key | FQYBTYFKOHPWQT-VGSWGCGISA-N | |
| InChi Code | InChI=1S/C24H27N3O5/c1-17(28)22(24(30)26-31)25-23(29)21-10-8-19(9-11-21)3-2-18-4-6-20(7-5-18)16-27-12-14-32-15-13-27/h4-11,17,22,28,31H,12-16H2,1H3,(H,25,29)(H,26,30)/t17-,22+/m1/s1 | |
| Chemical Name | N-[(2S,3R)-3-hydroxy-1-(hydroxyamino)-1-oxobutan-2-yl]-4-[2-[4-(morpholin-4-ylmethyl)phenyl]ethynyl]benzamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Escherichia coli LpxC(Ki= 4 nM) |
| ln Vitro | Disk diffusion assays have demonstrated that CHIR-090 has excellent antibiotic activity against P. aeruginosa and E. coli. It is a potent, slow, tight-binding inhibitor of the LpxC deacetylase from the hyperthermophile Aquifex aeolicus. Another two-step slow, tight-binding inhibitor of Escherichia coli LpxC with a Ki value of 4 nM is CHIR-090. Low nanometer concentrations of CHIR-090 inhibit LpxC orthologues from a variety of Gram-negative pathogens, such as Helicobacter pylori, Neisseria meningitidis, and Pseudomonas aeruginosa.On the other hand, Rhizobium leguminosarum (Ki=340 nM), a Gram-negative plant endosymbiont that is resistant to this compound, is resistant to LpxC from CHIR-090, a relatively weak competitive and conventional inhibitor (lacking slow, tight-binding kinetics). An E. Coli construct resistant to CHIR-090 up to 100 μg/mL, or 400 times higher than the minimal inhibitory concentration for wild-type E. Coli, has the chromosomal lpxC gene substituted with R. leguminosarum lpxC. The highly effective, slow-acting, tight-binding inhibitor CHIR-090 inhibits Aquifex aeolicus LpxC, whose sequence is 31% identical to that of E. coli LpxC. Disk diffusion assays have demonstrated that CHIR-090 possesses antibiotic activity against E. coli and P. aeruginosa that is comparable to that of ciprofloxacin[1]. |
| ln Vivo | Strong against E. coli, CHIR-090 suppresses E. coli LpxC activity in vitro at low nanometers (nM). Without prior chemical mutagenesis, E. Coli W3110 colonies resistant to 1 μg/mL CHIR-090 are not observed. On LB agar plates containing 1 to 10 μg/mL CHIR-090, a strain of E. coli W3110 can grow, exceeding the MIC of 0.25 μg/mL for wild-type E. coli W3110 by 4 to 40 times. In the presence of 1 μg/mL CHIR-090, W3110RL doubles in 40 minutes, which is precisely the same rate as wild-type in the absence of inhibitor. After roughly two hours in the presence of 1 μg/mL CHIR-090, wild-type cells stopped growing[1]. |
| Enzyme Assay | Disk diffusion is done, but each filter contains 10 μg of each antibiotic compound. Cells from overnight cultures are inoculated into 50 mL portions of LB broth at an A600 of 0.02 and grown with shaking at 30°C to assess growth in liquid medium in the presence of CHIR-090. Once the A600 reaches 0.15, 6 μL of 500 μg/mL CHIR-090 in DMSO or 6 μL of DMSO are added to the parallel cultures. When the A600 hits0.4, cultures are kept in log phase growth by 10-fold dilution into pre-warmed medium with the same concentrations of DMSO or DMSO/CHIR-090. This allows for the assessment of cumulative growth. When inoculating at an initial density of A600=0.01, the minimal inhibitory concentration is the lowest antibiotic concentration at which no detectable bacterial growth is seen in LB medium containing 1% DMSO (v/v). Cultures are incubated with CHIR-090 for 24 hours at 30°C while being shaken. Triplicates of each experiment are carried out[1]. |
| Cell Assay |
Antimicrobial susceptibility of biofilm-embedded cells (MBEC). [3] The minimum biofilm eradication concentration (MBEC) values of colistin and CHIR-090 against P. aeruginosa biofilms were evaluated in triplicate, as described by Naparstek et al.. Briefly, biofilms were grown in 96-well microplates for 24 h at 37°C in MH medium and then washed twice with 0.9% NaCl to remove the planktonic cells. Attached biofilms were then subjected to treatment with combinations of different antimicrobial concentrations in a checkerboard assay format. The antimicrobial concentrations tested varied from 0 to 512 and 0 to 128 μg/ml for colistin and CHIR-090, respectively. The plates were incubated for 24 h at 37°C. After antimicrobial challenge, the wells were thoroughly washed with 0.9% NaCl to remove any antimicrobial residue. Two hundred microliters of fresh MH growth medium was added to the washed wells, and then the plates were incubated for 24 h at 37°C. MBEC values were determined to be the lowest antibiotic concentrations that prevented bacterial growth from the treated biofilm. The potential for synergy was also evaluated by calculating the index of the fractional inhibitory concentration (ΣFIC) from the resulting biofilm checkerboard assay. All experiments were performed in triplicate. |
| Animal Protocol |
Mouse biofilm implant model of infection. [3] Seven-week-old female BALB/c mice were used in this study. The experimental setup and preparation were conducted on the basis of the protocol described by Chua et al. In summary, PAO1 biofilms were grown on cylindrical implants (3 mm by 5 mm in diameter) in 0.9% NaCl at 37°C with shaking at 110 rpm for 20 h. After incubation, biofilm-coated implants were washed three times with 0.9% NaCl and transplanted into the peritoneum of anesthetized mice. Antibiotic regimens were injected as a single dose at the implantation site of groups of five mice. Treatments and the associated groups included the control group, which was treated with no antibiotic (injection of 0.2 ml of 0.04% DMSO); test group 1, which was treated with 10 mg kg−1 of body weight colistin (which is well below the lethal dose of 86 mg kg−1 in mice); test group 2, which was treated with 4 mg kg−1 CHIR-090; and test group 3, which was treated with 10 mg kg−1 colistin and 4 mg kg−1 CHIR-090. After 24 h, mice were euthanized and the implants were retrieved from the peritoneum and washed with 0.9% NaCl. The implants were then sonicated in an ice water bath using an Elmasonic P120H sonicator at 50% power and 37 KHz for 10 min, followed by vortexing of the samples three times for 10 s each time. The spleens were also collected and homogenized using a Bio-Gen PRO200 homogenizer (Pro Scientific, USA) at maximum power on ice. The implants and spleen tissue samples were then serially diluted in 0.9% NaCl, plated onto LB agar, and incubated overnight at 37°C. The number of CFU was calculated and plotted versus treatment using Prism (version 7.0) software). Results are presented as means ± standard deviations. |
| References |
[1]. Inhibition of lipid A biosynthesis as the primary mechanism of CHIR-090 antibiotic activity in Escherichia coli. Biochemistry. 2007 Mar 27;46(12):3793-802. [2]. Structure of the deacetylase LpxC bound to the antibiotic CHIR-090: Time-dependent inhibition and specificity in ligand binding. Proc Natl Acad Sci U S A. 2007 Nov 20;104(47):18433-8. [3]. In Vitro and In Vivo Efficacy of an LpxC Inhibitor, CHIR-090, Alone or Combined with Colistin against Pseudomonas aeruginosa Biofilm. Antimicrob Agents Chemother. 2017 Jun 27;61(7). pii: e02223-16. |
| Additional Infomation | CHIR-090 is an L-threonine derivative obtained by formal condensation of the carboxy group of 4-({4-[(morpholin-4-yl)methyl]phenyl}ethynyl)benzoic acid with the amino group of N-hydroxy-L-threoninamide. It has a role as an antimicrobial agent, a lipopolysaccharide biosynthesis inhibitor and an EC 3.5.1.108 (UDP-3-O-acyl-N-acetylglucosamine deacetylase) inhibitor. It is an acetylenic compound, a member of morpholines, a member of benzamides, a L-threonine derivative and a hydroxamic acid. |
Solubility Data
| Solubility (In Vitro) | DMSO : 4~5 mg/mL ( 9.14~11.43 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 0.5 mg/mL (1.14 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 5.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 0.5 mg/mL (1.14 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 5.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 0.5 mg/mL (1.14 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 5.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 0.5 mg/mL (1.14 mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2858 mL | 11.4288 mL | 22.8577 mL | |
| 5 mM | 0.4572 mL | 2.2858 mL | 4.5715 mL | |
| 10 mM | 0.2286 mL | 1.1429 mL | 2.2858 mL |