CCG-1423 (CCG1423; CCG 1423) is a novel, potent and specific small-molecule inhibitor of RhoA signaling pathway with potential antitumor activity. It prevents transcription mediated by SRF. Several in vitro cancer cell functional assays show activity for CCG-1423. In PC-3 prostate cancer cells, CCG-1423 potently (<1 mumol/L) inhibits lysophosphatidic acid-induced DNA synthesis. At nanomolar concentrations, it also inhibits the growth of melanoma lines (A375M2 and SK-Mel-147) that overexpress RhoC, but it is less effective on related lines (A375 and SK-Mel-28) that express less Rho.
Physicochemical Properties
| Molecular Formula | C18H13CLF6N2O3 | |
| Molecular Weight | 454.75 | |
| Exact Mass | 454.051 | |
| Elemental Analysis | C, 47.54; H, 2.88; Cl, 7.80; F, 25.07; N, 6.16; O, 10.55 | |
| CAS # | 285986-88-1 | |
| Related CAS # | (S)-CCG-1423;2319939-24-5 | |
| PubChem CID | 2726015 | |
| Appearance | White to off-white solid powder | |
| Density | 1.5±0.1 g/cm3 | |
| Index of Refraction | 1.525 | |
| LogP | 6.59 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 9 | |
| Rotatable Bond Count | 5 | |
| Heavy Atom Count | 30 | |
| Complexity | 586 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | ClC1C([H])=C([H])C(=C([H])C=1[H])N([H])C(C([H])(C([H])([H])[H])ON([H])C(C1C([H])=C(C(F)(F)F)C([H])=C(C(F)(F)F)C=1[H])=O)=O |
|
| InChi Key | DSMXVSGJIDFLKP-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C18H13ClF6N2O3/c1-9(15(28)26-14-4-2-13(19)3-5-14)30-27-16(29)10-6-11(17(20,21)22)8-12(7-10)18(23,24)25/h2-9H,1H3,(H,26,28)(H,27,29) | |
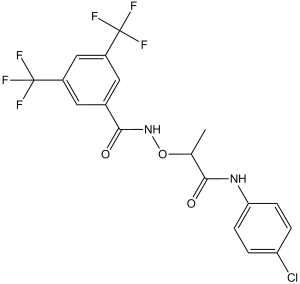
| Chemical Name | N-[1-(4-chloroanilino)-1-oxopropan-2-yl]oxy-3,5-bis(trifluoromethyl)benzamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Rho-pathway selective serum response element-luciferase reporter (IC50 = 1.5 µM) | ||
| ln Vitro |
|
||
| ln Vivo |
Pharmacological SRF inhibition by CCG-1423 reduced nuclear MKL1 and improved glucose uptake and tolerance in insulin-resistant mice in vivo. [6] The effect of an MKL1 inhibitor CCG-142330 on IRI was investigated in mice. When the mice were injected peritoneally with CCG-1423 for 3 days before the IR procedure, CCG-1423 injection resulted in a significant reduction of infarct size but did not afford detectable improvements in heart function (Figure I). When the mice were injected daily with CCG-1423 for 2 consecutive weeks before the IR procedure and found that prolonged pretreatment with CCG-1423 not only alleviated myocardial infarction (Figure 1E) but mitigated the loss of heart function (Figure 1F through 1H). This discrepancy in the effectiveness of 2 CCG regimens could be partly explained by the observation that although 2 weeks of CCG injection almost completely blocked the nuclear accumulation of MKL1 in cardiac macrophages compared with the vehicle group, 3 days of injection only marginally altered MKL1 localization (Figure II). Taken together, these data suggest that MKL1 loss of function might attenuate myocardial infarction and help retrieve the loss of heart function after IRI. [5] |
||
| Enzyme Assay | CCG-1423 is selective for Rho-overexpressing and invasive cancer cell lines, showing nanomolar to low micromolar potency in inhibiting DNA synthesis, cell growth, and/or invasion. Whereas the parental A375 cell line showed a smaller increase in Caspase-3 activation, daunorubicin showed the exact opposite pattern in the highly metastatic RhoC-overexpressing A375M2 melanoma cell line. | ||
| Cell Assay | In a 96-well plate coated with laminin, 2,000 cells in normal culture medium are plated per well. Following attachment, the medium is changed to serum-free medium (0% FBS) containing 30 μmol/L LPA, either in combination with or without 300 nM CCG-1423. To guarantee that LPA and compound are present for the duration of the experiment, fresh LPA, either with or without CCG-1423, is added on day 5. On day eight, the wells are filled with WST-1 reagent for one hour, and a Victor plate reader is used to measure the absorbance at 450 nm. | ||
| Animal Protocol |
|
||
| References |
[1].Mol Cancer Ther. 2007 Aug;6(8):2249-60. [2].Biochem Biophys Res Commun. 2010 Mar 19;393(4):877-82. [3].PLoS One. 2012;7(7):e40966. [4].Inflamm Bowel Dis. 2014 Jan;20(1):154-65. [5]. Circulation. 2018 Dec 11;138(24):2820-2836.[6]. J Clin Invest. 2011 Mar;121(3):918-29. doi: 10.1172/JCI41940. |
||
| Additional Infomation |
Lysophosphatidic acid receptors stimulate a Galpha(12/13)/RhoA-dependent gene transcription program involving the serum response factor (SRF) and its coactivator and oncogene, megakaryoblastic leukemia 1 (MKL1). Inhibitors of this pathway could serve as useful biological probes and potential cancer therapeutic agents. Through a transcription-based high-throughput serum response element-luciferase screening assay, we identified two small-molecule inhibitors of this pathway. Mechanistic studies on the more potent CCG-1423 show that it acts downstream of Rho because it blocks SRE.L-driven transcription stimulated by Galpha(12)Q231L, Galpha(13)Q226L, RhoA-G14V, and RhoC-G14V. The ability of CCG-1423 to block transcription activated by MKL1, but not that induced by SRF-VP16 or GAL4-VP16, suggests a mechanism targeting MKL/SRF-dependent transcriptional activation that does not involve alterations in DNA binding. Consistent with its role as a Rho/SRF pathway inhibitor, CCG-1423 displays activity in several in vitro cancer cell functional assays. CCG-1423 potently (<1 mumol/L) inhibits lysophosphatidic acid-induced DNA synthesis in PC-3 prostate cancer cells, and whereas it inhibits the growth of RhoC-overexpressing melanoma lines (A375M2 and SK-Mel-147) at nanomolar concentrations, it is less active on related lines (A375 and SK-Mel-28) that express lower levels of Rho. Similarly, CCG-1423 selectively stimulates apoptosis of the metastasis-prone, RhoC-overexpressing melanoma cell line (A375M2) compared with the parental cell line (A375). CCG-1423 inhibited Rho-dependent invasion by PC-3 prostate cancer cells, whereas it did not affect the Galpha(i)-dependent invasion by the SKOV-3 ovarian cancer cell line. Thus, based on its profile, CCG-1423 is a promising lead compound for the development of novel pharmacologic tools to disrupt transcriptional responses of the Rho pathway in cancer.[1] Embryonic stem cells (ESCs) are potentially powerful tools for regenerative medicine and establishment of disease models. The recent progress in ESC technologies is noteworthy, but ESC differentiation into renal lineages is relatively less established. The present study aims to differentiate mouse ESCs (mESCs) into a renal progenitor pool, the intermediate mesoderm (IM), without addition of exogenous cytokines and embryoid formation. First, we treated mESCs with a combination of small molecules (Janus-associated tyrosine kinase inhibitor 1, LY294002, and CCG1423) and differentiated them into BMP7-positive cells, BMP7 being the presumed inducing factor for IM. When these cells were cultured with adding retinoic acid, expression of odd-skipped related 1 (Osr1), which is essential to IM differentiation, was enhanced. To simplify the differentiation protocol, the abovementioned four small molecules (including retinoic acid) were combined and added to the culture. Under this condition, more than one-half of the cells were positive for Osr1, and at the same time, Pax2 (another IM marker) was detected by real-time PCR. Expressions of ectodermal marker and endodermal marker were not enhanced, while mesodermal marker changed. Moreover, expression of genes indispensable to kidney development, i.e., Lim1 and WT1, was detected by RT-PCR. These results indicate the establishment of a specific, effective method for differentiation of the ESC monolayer into IM using a combination of small molecules, resulting in an attractive cell source that could be experimentally differentiated to understand nephrogenic mechanisms and cell-to-cell interactions in embryogenesis.[2] Insulin resistance in skeletal muscle is a key phenotype associated with type 2 diabetes (T2D) for which the molecular mediators remain unclear. We therefore conducted an expression analysis of human muscle biopsies from patients with T2D; normoglycemic but insulin-resistant subjects with a parental family history (FH(+)) of T2D; and family history-negative control individuals (FH(–)). Actin cytoskeleton genes regulated by serum response factor (SRF) and its coactivator megakaryoblastic leukemia 1 (MKL1) had increased expression in T2D and FH(+) groups. Furthermore, striated muscle activator of Rho signaling (STARS), an activator of SRF, was upregulated in T2D and FH(+) and was inversely correlated with insulin sensitivity. Skeletal muscle from insulin-resistant mice recapitulated this gene expression pattern and showed reduced G-actin and increased nuclear localization of MKL1, each of which regulates SRF activity. Overexpression of MKL1 or reduction in G-actin decreased insulin-stimulated Akt phosphorylation, whereas reduction of STARS expression increased insulin signaling and glucose uptake. Pharmacological SRF inhibition by CCG-1423 reduced nuclear MKL1 and improved glucose uptake and tolerance in insulin-resistant mice in vivo. Thus, SRF pathway alterations are linked to insulin resistance, may contribute to T2D pathogenesis, and could represent therapeutic targets.[6] |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (5.50 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (5.50 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1990 mL | 10.9951 mL | 21.9901 mL | |
| 5 mM | 0.4398 mL | 2.1990 mL | 4.3980 mL | |
| 10 mM | 0.2199 mL | 1.0995 mL | 2.1990 mL |