CCF-642 is a protein disulfide isomerase (PDI) inhibitor. In 10 of 10 multiple myeloma cell lines, CCF642 showed a submicromolar IC50. Compared to the structurally distinct established inhibitors PACMA 31 and LOC14, CCF642 had a 100-fold greater inhibitory effect on PDI reductase activity in vitro. CCF642 demonstrated strong efficacy in an aggressive syngeneic mouse model of multiple myeloma and increased the life expectancy of C57BL/KaLwRij mice engrafted with 5TGM1-luc myeloma, exhibiting effects similar to those of the first-line multiple myeloma treatment bortezomib.
Physicochemical Properties
| Molecular Formula | C15H10N2O4S3 |
| Molecular Weight | 378.44 |
| Exact Mass | 377.98 |
| Elemental Analysis | C, 47.61; H, 2.66; N, 7.40; O, 16.91; S, 25.42 |
| CAS # | 346640-08-2 |
| Related CAS # | 346640-08-2 |
| PubChem CID | 1820764 |
| Appearance | Brown solid powder |
| Density | 1.6±0.1 g/cm3 |
| Boiling Point | 567.3±60.0 °C at 760 mmHg |
| Flash Point | 296.9±32.9 °C |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.760 |
| LogP | 4.11 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 3 |
| Heavy Atom Count | 24 |
| Complexity | 570 |
| Defined Atom Stereocenter Count | 0 |
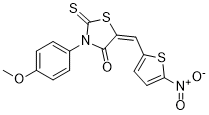
| SMILES | O=C1N(C2=CC=C(OC)C=C2)C(S/C1=C/C3=CC=C([N+]([O-])=O)S3)=S |
| InChi Key | SPYIETQLOVDJCF-XYOKQWHBSA-N |
| InChi Code | InChI=1S/C15H10N2O4S3/c1-21-10-4-2-9(3-5-10)16-14(18)12(24-15(16)22)8-11-6-7-13(23-11)17(19)20/h2-8H,1H3/b12-8+ |
| Chemical Name | (5E)-3-(4-methoxyphenyl)-5-[(5-nitrothiophen-2-yl)methylidene]-2-sulfanylidene-1,3-thiazolidin-4-one |
| Synonyms | CCF-642; CCF642; CCF 642 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | PDI (IC50 = 2.9 μM) |
| ln Vitro | CCF642 is about 100-fold more potent than the structurally distinct established inhibitors PACMA 31 and LOC14 at inhibiting PDI reductase activity in vitro. A novel covalent binding mode in active-site CGHCK motifs is suggested by computational modeling. In multiple myeloma cells, CCF642 induces calcium release that promotes apoptosis in addition to acute ER stress[1]. |
| ln Vivo | CCF642 exhibits strong efficacy in an aggressive syngeneic mouse model of multiple myeloma and extends the life of C57BL/KaLwRij mice engrafted with 5TGM1-luc myeloma, an effect similar to the first-line multiple myeloma treatment bortezomib[1]. |
| References |
[1]. Novel Protein Disulfide Isomerase Inhibitor with Anticancer Activity in Multiple Myeloma. Cancer Res. 2016 Jun 1;76(11):3340-50. |
Solubility Data
| Solubility (In Vitro) | DMSO: ≥ 30 mg/mL (~79.3 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: 0.62 mg/mL (1.64 mM) in 10% DMSO + 40% PEG300 +5% Tween-80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 6.2 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 + to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6424 mL | 13.2121 mL | 26.4243 mL | |
| 5 mM | 0.5285 mL | 2.6424 mL | 5.2849 mL | |
| 10 mM | 0.2642 mL | 1.3212 mL | 2.6424 mL |