CAY10603 is a novel, potent and selective HDAC6 (Histone deacetylase) inhibitor with potential anticancer activity and a potential to treat neurodegenerative diseases. It showed >200-fold selectivity over other HDACs and inhibits HDAC6 with an IC50 of 2 pM. The IC50 values of CAY10603 for HDAC1, 2, 3, 8, and 10 are 271 nM, 252 nM, 0.42 nM, 6851 nM, and 90.7 nM, in that order. For pancreatic cancer cell lines, CAY10603 exhibits strong antiproliferative activity with an IC50 of less than 1 μM. For researching HDAC biology, it might be a helpful chemical probe. Lung adenocarcinoma cell proliferation was inhibited and apoptosis was induced by CAY10603's inhibition of HDAC6. The EGFR signaling pathway was not activated when CAY10603 reduced the amounts of EGFR protein. Furthermore, CAY10603 and gefitinib worked together to destabilize EGFR, which caused the lung adenocarcinoma cell lines to undergo apoptosis. All of our findings point to the possibility that inhibiting HDAC6 could be a successful treatment method for lung adenocarcinoma.
Physicochemical Properties
| Molecular Formula | C22H30N4O6 | |
| Molecular Weight | 446.5 | |
| Exact Mass | 446.216 | |
| Elemental Analysis | C, 59.18; H, 6.77; N, 12.55; O, 21.50 | |
| CAS # | 1045792-66-2 | |
| Related CAS # |
|
|
| PubChem CID | 24951314 | |
| Appearance | White to light yellow solid powder | |
| Density | 1.2±0.1 g/cm3 | |
| Index of Refraction | 1.563 | |
| LogP | 1.94 | |
| Hydrogen Bond Donor Count | 4 | |
| Hydrogen Bond Acceptor Count | 7 | |
| Rotatable Bond Count | 12 | |
| Heavy Atom Count | 32 | |
| Complexity | 616 | |
| Defined Atom Stereocenter Count | 0 | |
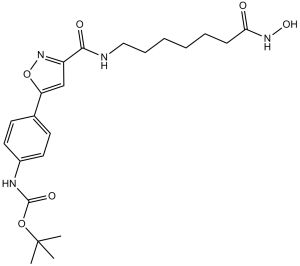
| SMILES | O(C(N([H])C1C([H])=C([H])C(=C([H])C=1[H])C1=C([H])C(C(N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C(N([H])O[H])=O)=O)=NO1)=O)C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H] |
|
| InChi Key | WWGBHDIHIVGYLZ-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C22H30N4O6/c1-22(2,3)31-21(29)24-16-11-9-15(10-12-16)18-14-17(26-32-18)20(28)23-13-7-5-4-6-8-19(27)25-30/h9-12,14,30H,4-8,13H2,1-3H3,(H,23,28)(H,24,29)(H,25,27) | |
| Chemical Name | tert-butyl N-[4-[3-[[7-(hydroxyamino)-7-oxoheptyl]carbamoyl]-1,2-oxazol-5-yl]phenyl]carbamate | |
| Synonyms | CAY-10603; CAY10603; BML-281; tert-Butyl (4-(3-((7-(hydroxyamino)-7-oxoheptyl)carbamoyl)isoxazol-5-yl)phenyl)carbamate; CAY-10603; tert-butyl N-[4-[3-[[7-(hydroxyamino)-7-oxoheptyl]carbamoyl]-1,2-oxazol-5-yl]phenyl]carbamate; CHEMBL511749; compound 3 [PMID: 18642892]; CAY 10603 | |
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | HDAC6 ( IC50 = 0.002 nM ); HDAC3 ( IC50 = 0.42 nM ); HDAC10 ( IC50 = 90.7 nM ); HDAC2 ( IC50 = 252 nM ); HDAC1 ( IC50 = 271 nM ); HDAC8 ( IC50 = 6851 nM ) | |
| ln Vitro |
|
|
| ln Vivo | HDAC6 was significantly upregulated in renal tubular epithelial cells (RTECs) of both AKI and CKD patients as well as mice. In the murine models of AKI induced by LPS and adenine-induced nephropathy, CAY10603 exhibited notable protective effects, including improvement in biochemical indices and pathological changes. In vivo and in vitro studies revealed that CAY10603 effectively suppressed the activation of activating transcription factor 6 (ATF6) branch of UPR triggered by thapsigargin (Tg), a commonly employed endoplasmic reticulum (ER) stressor. Consistent with these findings, CAY10603 also displayed substantial inhibition of ATF6 activation in RTECs from both murine models of LPS-induced AKI and adenine-induced nephropathy.Conclusions: Collectively, these results suggest that CAY10603 holds promise as a potential therapeutic agent for both acute and chronic kidney injury.[3] | |
| Enzyme Assay | The acetylated peptide substrate and test compound, labeled with 1 mm carboxyfluorescein (FAM), are incubated with purified HDACs for 17 hours at 25°C in an HDAC assay buffer that contains 100 mm HEPES (pH 7.5), 25 mm KCl, 0.1% BSA, and 0.01% Triton X-100. The addition of buffer containing 0.078% SDS for a final SDS concentration of 0.05% ends the reaction. Using a Caliper LabChip 3000 system with blue laser excitation and green fluorescence detection (CCD2), the substrate and product are separated electrophoretically. The Caliper system's Well Analyzer software is used to calculate the fluorescence intensity in the substrate and product peaks. For every sample, the reactions are carried out in duplicate. The XLFit 4-Parameter Logistic Model (sigmoidal dose-response model) and the IDBS XLFit version 4.2.1 plug-in for Microsoft Excel are used to automatically calculate IC50 values: ((A+ ((B_A)/1+((C/x)D)))), where x is the compound concentration, A and B denote the estimated minimum and maximum percent inhibition, respectively, C is the sigmoidal curve's inflection point, and D is its hill slope. | |
| Cell Assay | The ATCC provides the pancreatic cancer cell lines BxPc-3, HupT3, Mia Paca-2, Panc 04.03, and SU 86.86. The cell lines are cultured in DMEM or RPMI medium supplemented with 10% fetal calf serum and l-glutamine. In six wells of a 96-well microtiter plate, duplicate pancreatic cancer cells are plated out at a density of 2.5–4P103 cells per well. Individual wells are treated with diluent (DMSO), different concentrations of SAHA, or the specified HDACIs at a concentration of 1 nm to 50 mm four hours after plating. The colorimetric MTT assay is used to measure cytotoxicity at time "0" and 72 hours after treatment. XLfit is used to compute the IC50 values. | |
| Animal Protocol | Histone deacetylase 6 (HDAC6) inhibitor CAY10603 has been identified as a potential therapeutic agent for the treatment of diabetic kidney disease (DKD). The objective of this study was to investigate the therapeutic effects of CAY10603 in mice with acute kidney injury (AKI) and chronic kidney diseases (CKD). Methods: Renal immunohistology was performed to assess the expression levels of HDAC6 in both human and mouse kidney samples. C57BL/6J mice were intraperitoneal injected with lipopolysaccharide (LPS) to induce AKI; CD-1 mice were fed with adenine diet to induce adenine-nephropathy as CKD model. Serum creatinine, blood urea nitrogen and uric acid were measured to reflect renal function; renal histology was applied to assess kidney damage. Western blot and immunohistology were used to analyze the unfolded protein response (UPR) level.[3] | |
| References |
[1]. Use of the nitrile oxide cycloaddition (NOC) reaction for molecular probe generation: a new class of enzyme selective histone deacetylase inhibitors (HDACIs) showing picomolar activity at HDAC6. J Med Chem. 2008 Aug 14;51(15):4370-3. [2]. HDAC6 promotes cell proliferation and confers resistance to gefitinib in lung adenocarcinoma. Oncol Rep. 2016 Jul;36(1):589-97. [3]. Inhibition of HDAC6 with CAY10603 alleviates acute and chronic kidney injury by suppressing the ATF6 branch of UPR. Arch Biochem Biophys . 2024 Jun:756:110009. |
|
| Additional Infomation |
N-[4-[3-[[[7-(hydroxyamino)-7-oxoheptyl]amino]-oxomethyl]-5-isoxazolyl]phenyl]carbamic acid tert-butyl ester is a carbamate ester. A series of hydroxamate based HDAC inhibitors containing a phenylisoxazole as the CAP group has been synthesized using nitrile oxide cycloaddition chemistry. An HDAC6 selective inhibitor having a potency of approximately 2 picomolar was identified. Some of the compounds were examined for their ability to block pancreatic cancer cell growth and found to be about 10-fold more potent than SAHA. This research provides valuable, new molecular probes for use in exploring HDAC biology.[1] Histone deacetylases (HDACs) are promising targets for cancer therapy, and first-generation HDAC inhibitors are currently in clinical trials for the treatment of cancer patients. HDAC6, which is a key regulator of many signaling pathways that are linked to cancer, has recently emerged as an attractive target for the treatment of cancer. In the present study, HDAC6 was found to be overexpressed in lung adenocarcinoma cell lines and was negatively correlated with the prognosis of patients with lung adenocarcinoma. Overexpression of HDAC6 promoted the proliferation of lung adenocarcinoma cells in a deacetylase activity-dependent manner. HDAC6 overexpression conferred resistance to gefitinib via the stabilization of epidermal growth factor receptor (EGFR). The inhibition of HDAC6 by CAY10603, a potent and selective inhibitor of HDAC6, inhibited the proliferation of lung adenocarcinoma cells and induced apoptosis. CAY10603 downregulated the levels of EGFR protein, which in turn inhibited activation of the EGFR signaling pathway. Moreover, CAY10603 synergized with gefitinib to induce apoptosis of the lung adenocarcinoma cell lines via the destabilization of EGFR. Taken together, our results suggest that the inhibition of HDAC6 may be a promising strategy for the treatment of lung adenocarcinoma.[2] A series of 2-phenylthiazole analogues were designed and synthesized as potential histone deacetylase 6 (HDAC6) inhibitors based on compound 12c (an HDAC6/tubulin dual inhibitor discovered by us recently) and CAY10603 (a known HDAC6 inhibitor). Among them, compound XP5 was the most potent HDAC6 inhibitor with an IC50 of 31 nM and excellent HDAC6 selectivity (SI = 338 for HDAC6 over HDAC3). XP5 also displayed high antiproliferative activity against various cancer cell lines including the HDACi-resistant YCC3/7 gastric cancer cells (IC50 = 0.16-2.31 μM), better than CAY10603. Further, XP5 (50 mg/kg) exhibited significant antitumor efficacy in a melanoma tumor model with a tumor growth inhibition (TGI) of 63% without apparent toxicity. Moreover, XP5 efficiently enhanced the in vivo antitumor immune response when combined with a small-molecule PD-L1 inhibitor, as demonstrated by the increased tumor-infiltrating lymphocytes and reduced PD-L1 expression levels. Taken together, the above results suggest that XP5 is a promising HDAC6 inhibitor deserving further investigation.J Med Chem. 2022 Feb 10;65(3):2434-2457. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (5.60 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (5.60 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (5.60 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 5% DMSO+50% PEG 300+ddH2O: 9mg/mL Solubility in Formulation 5: 5 mg/mL (11.20 mM) in 50% PEG300 50% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; Need ultrasonic and warming and heat to 42°C. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2396 mL | 11.1982 mL | 22.3964 mL | |
| 5 mM | 0.4479 mL | 2.2396 mL | 4.4793 mL | |
| 10 mM | 0.2240 mL | 1.1198 mL | 2.2396 mL |