Physicochemical Properties
| Molecular Formula | C18H12CL2N2O |
| Molecular Weight | 343.21 |
| Exact Mass | 342.032 |
| CAS # | 188425-85-6 |
| Related CAS # | Boscalid-d4;2468796-76-9 |
| PubChem CID | 213013 |
| Appearance | White to off-white solid powder |
| Density | 1.3±0.1 g/cm3 |
| Boiling Point | 557.0±60.0 °C at 760 mmHg |
| Melting Point | 142.8 to 143.8ºC |
| Flash Point | 290.7±32.9 °C |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.636 |
| LogP | 5.72 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 3 |
| Heavy Atom Count | 23 |
| Complexity | 399 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | WYEMLYFITZORAB-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C18H12Cl2N2O/c19-13-9-7-12(8-10-13)14-4-1-2-6-16(14)22-18(23)15-5-3-11-21-17(15)20/h1-11H,(H,22,23) |
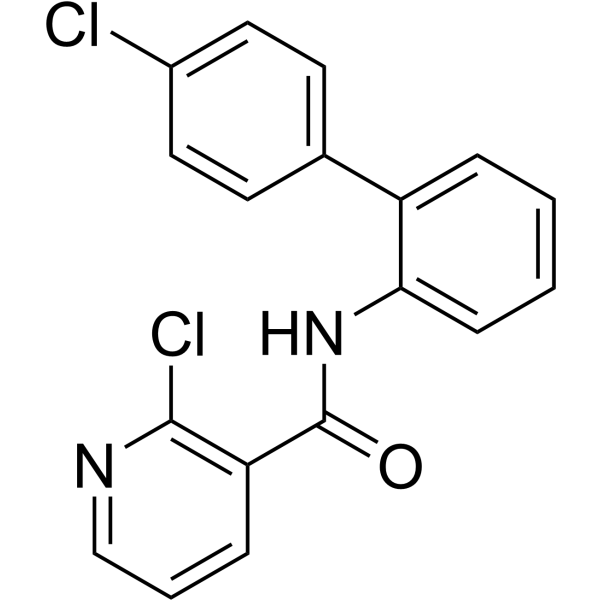
| Chemical Name | 2-chloro-N-[2-(4-chlorophenyl)phenyl]pyridine-3-carboxamide |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | succinate dehydrogenase (SDH)[1] |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Dermal Penetration (rat). Maximum % absorption: 0.01 mg/sq cm = 10.93 (24 hour exposure, 24 hour sacrifice) 0.10 mg/sq cm = 3.76 (24 hour exposure, 24 hour sacrifice) 1.00 mg/sq cm = 1.48 (10 hour exposure, 72 hour sacrifice /From table/ In the rat, Boscalid was readily absorbed and excreted following single oral 50 mg/kg; at single 500 mg/kg or 15 doses of 500 mg/kg, absorption was saturated. Excretion mainly by feces (80-98%). Biliary excretion 40- 50% of fecal activity at 50 mg/kg, 10% at 500 mg/kg. Urine, about 16% at 50 mg/kg, 3-5% at 500 mg/kg. Absorption about 56% at 50 mg/kg and 13-17% at 500 mg/kg. Excretory patterns similar by gender or radiolabel position. /From table/ Metabolism / Metabolites Three ... groups of Wistar rats were treated and sampled ... for qualitative analyses of metabolites. ... Metabolites were separated by HPLC. Primary identification was by mass spectrometry (MS). ... The most important metabolites were hydroxyl or O-glucuronide metabolites on the diphenyl ring (usually para to the amide nitrogen), and S-glucuronide conjugation products displacing the chlorine on the pyridine ring of the parent compound. The sulfur originated from glutathione (GSH) addition to the ring. GSH was often cleaved to cysteine in bile or feces, or further degraded in feces to a thiol, which in turn was sometimes conjugated as a glucuronide). Tissue residues (liver, kidney, and plasma) were scant ... Some parent BAS 510 F was found in kidneys and plasma. Thus BAS 510 F was effectively metabolized and efficiently excreted. /In the rat,/ metabolites (hydroxylation and conjugation products) were consistent with Phase I oxidation reactions followed by Phase II conjugation with glucuronic acid or sulfate, or by conjugation of the parent with glutathione with cleavage to sulfate metabolites. /From table/ Biological Half-Life In the rat, the predominant route of excretion of BAS 510 F is fecal with urinary excretion being minor. The half-life of BAS 510 F is less than 24 hours. |
| Toxicity/Toxicokinetics |
Toxicity Summary IDENTIFICATION AND USE: Boscalid is a solid. It is used as fungicide, plant health product, seed treatment/protectant. HUMAN EXPOSURE AND TOXICITY: Boscalid may be genotoxic and cytotoxic in vitro in human peripheral blood lymphocytes. ANIMAL STUDIES: Boscalid has a low toxicity in animal studies. In subchronic and chronic feeding studies in rats, mice and dogs, boscalid generally caused decreased body weights and body weight gains and effects on the liver (increase in weights, changes in enzyme levels and histopathological changes) as well as on the thyroid (increase in weights and histopathological changes). In a developmental toxicity study in rats, no developmental toxicity was observed in the fetuses at the highest dose tested. In a developmental toxicity study in rabbits, an increased incidence of abortions or early delivery was observed at the limit dose. The does and fetuses were equally sensitive to the test material. In a 2-generation reproduction study in rats, the NOAEL for parental toxicity was based on decreased body weight and body weight gain as well as hepatocyte degeneration. No reproductive toxicity was observed in this study at the highest dose tested. There was quantitative evidence of increased susceptibility in the developmental neurotoxicity study in rats, where decreases in pup body weights and body weight gains were seen in the absence of any maternal toxicity. In a two-year chronic toxicity study and a two-year carcinogenicity study in male and female rats, the combined data showed that, for thyroid follicular cell adenomas, males had a significant increasing trend, when compared with controls. There was no treatment-related increase in thyroid follicular cell carcinomas. The increase in thyroid follicular cell adenomas appeared to be treatment-related in males. Regarding females, combined data from the two rat studies indicated that there was an increasing trend for thyroid follicular cell adenomas. No carcinomas were observed in female. Boscalid was tested in five mutagenicity studies and was found to be negative in all of them. ECOTOXICITY STUDIES: Boscalid is categorized as practically nontoxic to birds in both an acute and subacute studies. Boscalid was harmless to adult Galendromus occidentalis. Boscalid use does not represent a risk to plants. Commercial producers of honey bee queens (Apis mellifera L.) have reported unexplained loss of immature queens during the larval or pupal stage. Many affected queen-rearing operations are situated among the almond orchards of California and report these losses in weeks after almond trees bloom. Almond flowers are a rich foraging resource for bees, but are often treated with fungicides, insecticides, and spray adjuvants during bloom. Anecdotal reports by queen producers associate problems in queen development with application of the fungicide Pristine (boscalid and pyraclostrobin). Chemical analysis revealed that low concentrations of pyraclostrobin (50 ppb), but no boscalid, were detectable in royal jelly secreted by nurse bees feeding on treated pollen. Toxicity Data LC50 (rat) > 6,700 mg/m3 Non-Human Toxicity Values LD50 Rat oral >5,000 mg/kg (Technical boscalid) /From table/ LD50 Rat dermal >2,000 mg/kg (Technical boscalid) /From table/ |
| References |
[1]. Fungicide composition comprising pyridine carboxamide, strobilurin and dithiocarbamate. Patent. WO2023042225 A1. 2023-03-23. |
| Additional Infomation |
Boscalid is a pyridinecarboxamide obtained by formal condensation of the carboxy group of 2-chloronicotinic acid with the amino group of 4'-chlorobiphenyl-2-amine. A fungicide active against a broad range of fungal pathogens including Botrytis spp., Alternaria spp. and Sclerotinia spp. for use on a wide range of crops including fruit, vegetables and ornamentals. It has a role as an EC 1.3.5.1 [succinate dehydrogenase (quinone)] inhibitor, an environmental contaminant, a xenobiotic and an antifungal agrochemical. It is a member of biphenyls, a pyridinecarboxamide, a member of monochlorobenzenes and an anilide fungicide. It is functionally related to a nicotinic acid. Boscalid has been investigated for the treatment of OSDI, Glaucoma, Staining, Schirmers, and Disease Severity, among others. Boscalid has been reported in Ganoderma lucidum with data available. Boscalid is a fungicide developed by BASF and launched in 2003 for use on food crops. It works as a succinate dehydrogenase inhibitor to kill fungal target organisms. It is practically nontoxic to terrestrial animals and is moderately toxic to aquatic animals on an acute exposure basis. In subchronic and chronic feeding studies in rats, mice and dogs, boscalid generally caused decreased body weights and body weight gains (primarily in mice) and effects on the liver (increase in weights, changes in enzyme levels and histopathological changes) as well as on the thyroid (increase in weights and histopathological changes). In a developmental toxicity study in rats, no developmental toxicity was observed in the fetuses at the highest dose tested. Boscalid is classified as, suggestive evidence of carcinogenicity, but not sufficient to assess human carcinogenic potential, according to the EPA. |
Solubility Data
| Solubility (In Vitro) | DMSO : 100 mg/mL (291.37 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (7.28 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (7.28 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9137 mL | 14.5683 mL | 29.1367 mL | |
| 5 mM | 0.5827 mL | 2.9137 mL | 5.8273 mL | |
| 10 mM | 0.2914 mL | 1.4568 mL | 2.9137 mL |