Physicochemical Properties
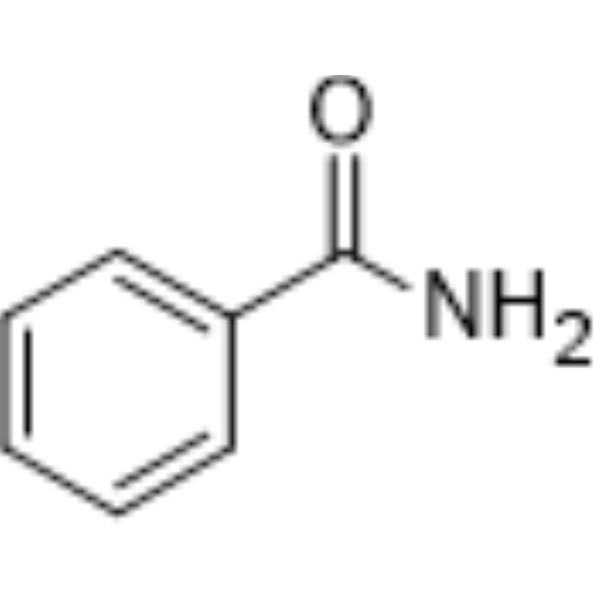
| Molecular Formula | C7H7NO |
| Molecular Weight | 121.137 |
| Exact Mass | 121.052 |
| CAS # | 55-21-0 |
| Related CAS # | 55738-52-8 |
| PubChem CID | 2331 |
| Appearance | White to light yellow solid powder |
| Density | 1.1±0.1 g/cm3 |
| Boiling Point | 221.5±23.0 °C at 760 mmHg |
| Melting Point | 125-128 °C(lit.) |
| Flash Point | 87.8±22.6 °C |
| Vapour Pressure | 0.1±0.5 mmHg at 25°C |
| Index of Refraction | 1.547 |
| LogP | 0.84 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Heavy Atom Count | 9 |
| Complexity | 106 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | KXDAEFPNCMNJSK-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C7H7NO/c8-7(9)6-4-2-1-3-5-6/h1-5H,(H2,8,9) |
| Chemical Name | benzamide |
| Synonyms | Benzenecarboxamide; Benzamide; Phenylamide |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Human Endogenous Metabolite |
| ln Vitro | Benzamide, an inhibitor of PARP protects primary cultures of neurons derived from neonatal rat brain against cell death caused by excitatory amino acids. Furthermore, it has recently been demonstrated that benzamide can at least partially shield fetal rat mesencephalic cells in culture from the in vitro effects of METH, including the loss of dopaminergic cells and an increase in reactive gliosis[1]. Benzamide inhibits transformation in a way that is specific to the cell cycle, with the greatest prevention occurring in the early S phase, which is also a feature of the maximum susceptibility to transformation[2]. |
| ln Vivo | Benzamide, a PARP inhibitor, protects C57Bl/6N mice's brains from various neurotoxicities while maintaining body temperature[2]. Neuronal survival and memory during GCI are improved by benzoamide treatment, which dramatically reduces apoptotic neuron count and iNOS expression. Administering 160 mg/kg i.p. of benzamide does not cause hypothermia, and it reaches the central nervous system (CNS) in 30 minutes at concentrations between 0.09 and 0.64 mM, where it exhibits neuroprotection[3]. |
| Cell Assay | The release of the metabolically induced G1/S block is followed by three washings and refeeding with fresh media for ten hours, during which time the carcinogens and benzamide are exposed. |
| Animal Protocol |
C57B1/6N mice (intraperitoneal injection of METH at 2-h intervals; 4 injections of 5 mg/kg, 4 injections of 10 mg/kg, or 2 injections of 20 mg/kg) 160 mg/kg IP, 2 injection by a 4 h interval |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion The percutaneous absorption of the fragrances benzyl acetate and five other benzyl derivatives (benzyl alcohol, benzyl benzoate, benzamide, benzoin and benzophenone) was determined in vivo in monkeys. Absorption through occluded skin was high for all compounds (approximately 70% of the applied dose in 24 hr) and no significant differences between values for the different compounds were observed. No correlations were seen between skin penetration of these compounds and their octanol-water partition coefficients. Under unoccluded conditions skin penetration of the fragrances was reduced and there was great variability between compounds, presumably because of variations in the rates of evaporation from the site of application. The data suggest that humans may have significant systemic exposure to these fragrance. Metabolism / Metabolites Benzamide yields benzoic acid in rabbit, guinea pig, dog, pig, & cat. /From table/ |
| Toxicity/Toxicokinetics |
Interactions Phorbol ester-induced promotion of initiated NMRI mouse skin keratinocytes to papillomas could be largely prevented when nicotinamide like inhibitors of poly(ADP-ribose)polymerase (nicotinamide, benzamide, 3-aminobenzamide) were applied simultaneously with 12-O-tetradecanoylphorbol-13-acetate. A similar suppression of tumor promotion by nicotinamide analogues was demonstrated in clone 41 JB6 epidermal cells which are promotable by 12-O-tetradecanoylphorbol- 13-acetate to anchorage-independent growth. Differences in the mode of action of caffeine and benzamide on DNA repair processes were studied in Chinese hamster lung fibroblast line V79 cells treated or not treated with N-methyl-N'-nitro-N-nitrosoguanidine. Post treatment with 2 mM caffeine or benzamide for 24 hr, following N-methyl-N'-nitro-N-nitrosoguanidine treatment, greatly increased cell lethality. In the presence of 50 uM deoxycytidine, caffeine had almost no effect on cell lethality. Benzamide induced cell lethality was not affected by the presence of deoxycytidine. Treatment with 2 mM caffeine or benzamide alone for 24 hours had no effect on cell lethality. When caffeine and benzamide were present simultaneously for 96 hr, cell lethality was greatly increased. This effect was eliminated in the presence of 50 uM deoxycytidine. Poly(ADP-ribose)polymerase activity of the cells was substantially inhibited by 2 mM benzamide; 2 to 5 mM caffeine had no detectable effect on the polymerase activity. Non-Human Toxicity Values LD50 Rat ip 781 mg/kg LD50 Mouse oral 1160 mg/kg |
| References |
[1]. Brain Res . 1996 Oct 7;735(2):343-8. [2]. Proc Natl Acad Sci U S A . 1983 Dec;80(23):7219-23. [3]. Behav Brain Res . 2008 Oct 10;192(2):178-84. [4]. J Biol Chem . 1989 Mar 15;264(8):4312-7. |
| Additional Infomation |
Benzamide is a white powder. (NTP, 1992) Benzamide is an aromatic amide that consists of benzene bearing a single carboxamido substituent. The parent of the class of benzamides. Benzamide has been reported in Streptomyces, Colchicum autumnale, and other organisms with data available. |
Solubility Data
| Solubility (In Vitro) |
DMSO : 24~120 mg/mL (198.1~990.6 mM) Ethanol : ~24 mg/mL (~198.1 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 3 mg/mL (24.76 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 30.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 3 mg/mL (24.76 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 30.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 3 mg/mL (24.76 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 30.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 8.2549 mL | 41.2746 mL | 82.5491 mL | |
| 5 mM | 1.6510 mL | 8.2549 mL | 16.5098 mL | |
| 10 mM | 0.8255 mL | 4.1275 mL | 8.2549 mL |