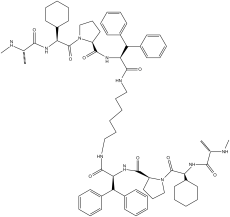
BV-6 is a novel and potent SMAC (second mitochondrial-derived activator of caspases) mimetic, and a dual inhibitor of cIAP (inhibitor of apoptosis) and XIAP (X-linked inhibitor of apoptosis) with potential anticancer activity. It works by stimulating the differentiation of glioblastoma cancer stem cells by turning on NF-κB.
Physicochemical Properties
| Molecular Formula | C70H96N10O8 | |
| Molecular Weight | 1205.57 | |
| Exact Mass | 1204.74 | |
| Elemental Analysis | C, 69.74; H, 8.03; N, 11.62; O, 10.62 | |
| CAS # | 1001600-56-1 | |
| Related CAS # |
|
|
| PubChem CID | 23657864 | |
| Appearance | White to off-white a crystalline solid | |
| LogP | 9.744 | |
| Hydrogen Bond Donor Count | 8 | |
| Hydrogen Bond Acceptor Count | 10 | |
| Rotatable Bond Count | 29 | |
| Heavy Atom Count | 88 | |
| Complexity | 2030 | |
| Defined Atom Stereocenter Count | 8 | |
| SMILES | O=C(C([H])(C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C1([H])[H])N([H])C(C([H])(C([H])([H])[H])N([H])C([H])([H])[H])=O)N1C([H])([H])C([H])([H])C([H])([H])C1([H])C(N([H])C([H])(C(N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])N([H])C(C([H])(C([H])(C1C([H])=C([H])C([H])=C([H])C=1[H])C1C([H])=C([H])C([H])=C([H])C=1[H])N([H])C(C1([H])C([H])([H])C([H])([H])C([H])([H])N1C(C([H])(C1([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C1([H])[H])N([H])C(C([H])(C([H])([H])[H])N([H])C([H])([H])[H])=O)=O)=O)=O)=O)C([H])(C1C([H])=C([H])C([H])=C([H])C=1[H])C1C([H])=C([H])C([H])=C([H])C=1[H])=O |
|
| InChi Key | DPXJXGNXKOVBJV-YLOPQIBLSA-N | |
| InChi Code | InChI=1S/C70H96N10O8/c1-47(71-3)63(81)75-59(53-37-21-11-22-38-53)69(87)79-45-27-41-55(79)65(83)77-61(57(49-29-13-7-14-30-49)50-31-15-8-16-32-50)67(85)73-43-25-5-6-26-44-74-68(86)62(58(51-33-17-9-18-34-51)52-35-19-10-20-36-52)78-66(84)56-42-28-46-80(56)70(88)60(54-39-23-12-24-40-54)76-64(82)48(2)72-4/h7-10,13-20,29-36,47-48,53-62,71-72H,5-6,11-12,21-28,37-46H2,1-4H3,(H,73,85)(H,74,86)(H,75,81)(H,76,82)(H,77,83)(H,78,84)/t47-,48-,55-,56-,59-,60-,61-,62-/m0/s1 | |
| Chemical Name | (2S)-1-[(2S)-2-cyclohexyl-2-[[(2S)-2-(methylamino)propanoyl]amino]acetyl]-N-[(2S)-1-[6-[[(2S)-2-[[(2S)-1-[(2S)-2-cyclohexyl-2-[[(2S)-2-(methylamino)propanoyl]amino]acetyl]pyrrolidine-2-carbonyl]amino]-3,3-diphenylpropanoyl]amino]hexylamino]-1-oxo-3,3-diphenylpropan-2-yl]pyrrolidine-2-carboxamide | |
| Synonyms | Smac mimetic BV6; BV6; BV-6; BV 6 | |
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | cIAP; XIAP | ||
| ln Vitro |
Inducing apoptosis in both HCC193 and H460 cell lines, BV6 also significantly increases the radiosensitivity of these cell lines by activating cleaved caspase-8 and cleaved caspase-9, respectively. BV6 inhibits the cell viability of HCC193 NSCLC cells with an IC50 of 7.2 μM. [1] The traditional NF-kB pathway is moderately activated in immature dendritic cells after BV-6 treatment.[2] Additionally, BV-6 increases the CIK cell-mediated lysis of solid malignancies (RH1, RH30, and TE671) as well as hematological malignancies (H9, THP-1, and Tanoue). Additionally, BV-6 increases the apoptosis of peripheral blood mononuclear cells and, most significantly, inhibits immune cells, reducing their capacity for cytotoxicity.[3] |
||
| ln Vivo | Murine cIAP-1, cIAP-2 and XIAP expressions are clearly observed in the cytoplasm of both epithelial and stromal cells of implants, whereas Survivin is mainly expressed in the nuclei BV6 treatment for 4 weeks attenuated the intensity of IAPs expression. Lesions can be anywhere between 2 and 7 mm in diameter. The cyst's monolayer epithelial cell lining is visible. Vimentin and cytokeratin are positively stained after immunohistochemical staining, but calretinin is negatively stained. The total number of lesions (4.6 versus 2.8/mouse), average weight (78.1 versus 32.0 mg/mouse), and surface area (44.5 versus 24.6 mm2/mouse) of lesions are all significantly lower than in the control group after 4 weeks of BV6 treatment. In the endometrial gland epithelia or stroma, the percentage of Ki67-positive cells decreases after BV6 treatment. | ||
| Cell Assay | The CellTiter 96® Aqueous Non-Radioactive Cell Proliferation Assay kit is used to assess cell viability. In triplicate, 96-well plates are seeded with 5000 cells per well. Different wells are then filled with BV6 in varying concentrations after the cells have adhered to them. The same amount of DMSO is administered to the control groups. 24 hours later, the final doses of 333 μg/mL MTS and 25 μM PMS are added to each well. Plates are read at 490 nm on a microplate reader after two hours of incubation at 37 °C in humidified 5% CO2. By comparing the absorbance of each sample to that of the corresponding control, one can determine the relative cell viability of each one. Using Prism 5.01, the IC50 values are determined. Cells are exposed to 1 and 5 μM BV6 with or without 10 μg/mL infliximab for the TNFα-neutralizing antibody assay, which is then conducted 24 hours later. Using a microplate reader, plates are read at 490 nm absorbance. | ||
| Animal Protocol |
|
||
| References |
[1]. J Thorac Oncol . 2011 Nov;6(11):1801-9. [2]. PLoS One . 2011;6(6):e21556. [3]. Front Pediatr . 2014 Jul 18:2:75. [4]. Hum Reprod . 2015 Jan;30(1):149-58. |
||
| Additional Infomation | N,N'-(hexane-1,6-diyl)bis(1-{(2S)-2-cyclohexyl-2-[(N-methyl-L-alanyl)amino]acetyl}-L-prolyl-beta-phenyl-L-phenylalaninamide) is a polyamide consisting of hexane-1,6-diamine having a 1-{(2S)-2-cyclohexyl-2-[(N-methyl-L-alanyl)amino]acetyl}-L-prolyl-beta-phenyl-L-phenylalanyl moiety attached to both nitrogens. It is functionally related to a methyl 1-{(2S)-2-cyclohexyl-2-[(N-methyl-L-alanyl)amino]acetyl}-L-prolyl-beta-phenyl-L-phenylalaninate. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (2.07 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (2.07 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (2.07 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.8295 mL | 4.1474 mL | 8.2948 mL | |
| 5 mM | 0.1659 mL | 0.8295 mL | 1.6590 mL | |
| 10 mM | 0.0829 mL | 0.4147 mL | 0.8295 mL |