BRD7116 is a novel, potent and selective Inhibitor of leukemia stem cell (LSC) activity with EC50 of 200 nM. It exhibits >100-fold selectivity for LSCs over normal hematopoietic stem cells. Efforts to develop more effective therapies for acute leukemia may benefit from high-throughput screening systems that reflect the complex physiology of the disease, including leukemia stem cells (LSCs) and supportive interactions with the bone marrow microenvironment. The therapeutic targeting of LSCs is challenging because LSCs are highly similar to normal hematopoietic stem and progenitor cells (HSPCs) and are protected by stromal cells in vivo.
Physicochemical Properties
| Molecular Formula | C28H36N2O4S |
| Molecular Weight | 496.66144657135 |
| Exact Mass | 496.239 |
| Elemental Analysis | C, 67.71; H, 7.31; N, 5.64; O, 12.89; S, 6.46 |
| CAS # | 329059-55-4 |
| PubChem CID | 981129 |
| Appearance | Solid powder |
| Density | 1.2±0.1 g/cm3 |
| Boiling Point | 681.1±40.0 °C at 760 mmHg |
| Flash Point | 365.7±27.3 °C |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.576 |
| LogP | 6.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 35 |
| Complexity | 859 |
| Defined Atom Stereocenter Count | 0 |
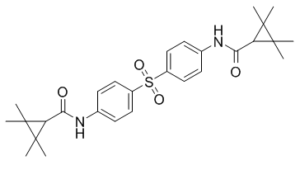
| SMILES | O=S(C1=CC=C(NC(C2C(C)(C)C2(C)C)=O)C=C1)(C3=CC=C(NC(C4C(C)(C)C4(C)C)=O)C=C3)=O |
| InChi Key | UHXFWAHRAMUDLJ-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C28H36N2O4S/c1-25(2)21(26(25,3)4)23(31)29-17-9-13-19(14-10-17)35(33,34)20-15-11-18(12-16-20)30-24(32)22-27(5,6)28(22,7)8/h9-16,21-22H,1-8H3,(H,29,31)(H,30,32) |
| Chemical Name | N,N'-(Sulfonyldi-4,1-phenylene)bis[2,2,3,3-tetramethylcyclopropanecarboxamide] |
| Synonyms | BRD7116; BRD-7116; BRD 7116. |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro |
BRD7116, a bis-aryl sulfone, showed evidence of stroma-mediated anti-LSCe activity. BRD7116 exhibited an EC50 of 200 nM for LSCe cells in co-culture, whereas it displayed limited activity against normal HSPCs and AML cell lines (~50% inhibition at 20 μM) . BRD7116 also showed activity against patient-derived, primary human leukemia cells . Furthermore, pretreatment of the stroma partially recapitulated the leukemic CAFC inhibition observed in co-culture without evidence of altered stromal cell viability (CellTiter-Glo). In contrast to troglitazone, this niche effect was not accompanied by an obvious change in stromal morphology.We next directly compared the relative inhibition of LSCe cells and HSPCs under internally controlled conditions using a 3-component co-culture system. We added dsRed+ LSCe cells and GFP+ HSPCs (from ubiquitin C-GFP mice) simultaneously to uncolored bone marrow stromal cells pretreated for 3 days with BRD7116. In contrast to DMSO pretreatment controls, BRD7116 pretreatment of the stroma preferentially inhibited the LSCe cells compared to HSPCs . Having observed a cell-non-autonomous effect, we next explored whether BRD7116 had any cell-autonomous effects on LSCe cells. We exposed LSCe cells for 6 hours to 5 μM BRD7116 in suspension, then performed gene-expression profiling. Using Gene Set Enrichment Analysis (GSEA)26,27, we found that treatment of LSCe cells with BRD7116 induced transcriptional changes consistent with myeloid differentiation, as defined by all-trans retinoic acid (ATRA) treatment of ATRA-sensitive human AML cells28 . The mechanism by which BRD7116 has this differentiation-inducing effect remains to be determined. |
| References |
[1]. Niche-based screening identifies small-molecule inhibitors of leukemia stem cells. Nat Chem Biol. 2013 Dec;9(12):840-848. |
| Additional Infomation | 2,2,3,3-tetramethyl-N-[4-[4-[[oxo-(2,2,3,3-tetramethylcyclopropyl)methyl]amino]phenyl]sulfonylphenyl]-1-cyclopropanecarboxamide is an anilide. |
Solubility Data
| Solubility (In Vitro) | DMSO : ≥ 48 mg/mL (~96.65 mM) |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0134 mL | 10.0672 mL | 20.1345 mL | |
| 5 mM | 0.4027 mL | 2.0134 mL | 4.0269 mL | |
| 10 mM | 0.2013 mL | 1.0067 mL | 2.0134 mL |