BIBR 1532 (BIBR-1532; BIBR1532) is a potent, highly selective, nonnucleosidic and non-competitive inhibitor of telomerase with potential anticancer activity. In a test without cells, it inhibits telomerase with an IC50 of 100 nM. When BIBR1132 inhibited telomerase, tumor cells experienced a delayed growth arrest. Following a delay that is mostly reliant on initial telomere length, treatment of cancer cells with BIBR1532 results in progressive telomere shortening, subsequent telomere dysfunction, and ultimately growth arrest. Since most cancer cells can proliferate indefinitely when telomerase is activated, it is a desirable target for mechanism-based therapeutic approaches.
Physicochemical Properties
| Molecular Formula | C21H17NO3 | |
| Molecular Weight | 331.36 | |
| Exact Mass | 331.12 | |
| Elemental Analysis | C, 76.12; H, 5.17; N, 4.23; O, 14.49 | |
| CAS # | 321674-73-1 | |
| Related CAS # |
|
|
| PubChem CID | 9927531 | |
| Appearance | White solid powder | |
| Density | 1.3±0.1 g/cm3 | |
| Boiling Point | 600.6±48.0 °C at 760 mmHg | |
| Flash Point | 317.0±29.6 °C | |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C | |
| Index of Refraction | 1.698 | |
| LogP | 6.31 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 3 | |
| Rotatable Bond Count | 4 | |
| Heavy Atom Count | 25 | |
| Complexity | 527 | |
| Defined Atom Stereocenter Count | 0 | |
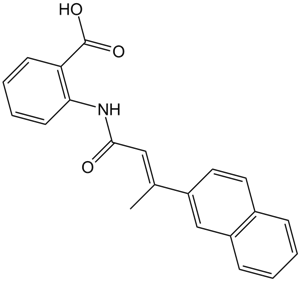
| SMILES | O=C(O)C1=CC=CC=C1NC(/C=C(C2=CC=C3C=CC=CC3=C2)\C)=O |
|
| InChi Key | PGFQXGLPJUCTOI-WYMLVPIESA-N | |
| InChi Code | InChI=1S/C21H17NO3/c1-14(16-11-10-15-6-2-3-7-17(15)13-16)12-20(23)22-19-9-5-4-8-18(19)21(24)25/h2-13H,1H3,(H,22,23)(H,24,25)/b14-12+ | |
| Chemical Name | 2-[[(E)-3-naphthalen-2-ylbut-2-enoyl]amino]benzoic acid | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | telomerase ( IC50 = 100 nM ) | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Enzyme Assay | In order to perform the direct telomerase assay using endogenous telomerase, 10 μL of telomerase-enriched extract and various BIBR1532 concentrations are combined into a final volume of 20 μL. 20 μL of the reaction mixture is added after a 15-minute ice preincubation period, and the tubes are then heated to 37°C to start the reaction. The reaction mixture's final concentrations are as follows: 1.25 mM spermidine, 10 units of RNasin, 5 mM 2-mercaptoethanol, 6.3 μM cold dGTP, 15 μCi [α-32P]dGTP (3000 Ci/mmol; NEN), 1 mM dATP, 1 mM dTTP, and 1 mM TS-primer (5'-AATCCGTCGAGCAGAGTT). For the recombinant enzyme, 1–7 μL of affinity-purified telomerase (with less than 0.025 μM hTERT) are tested in a final volume of 40 μL that includes 50 mM Tris acetate (pH 8.5), 50 mM KCl, 1 mM MgCl2, 1 mM spermidine, 5 mM 2-mercaptoethanol, 1 mM dATP, 1 mM dTTP, 2.5 μM dGTP, 15 μCi of [α-32P]dGTP (3000 Ci/mmol), and 2.5 μM (TTAGGG)3. The process begins with two hours of incubation at 37°C, and is terminated with the addition of 50 μL of RNase mix (0.1 mg/mL RNaseA-100 u/mL RNaseT1 in 10 mM Tris-Cl (pH 8.3) and 20 mm EDTA) and another 20 minutes of incubation at 37°C. To deproteinate samples, mix 50 μL of 0.3 mg/m proteinase K with 10 mM Tris-Cl (pH 8.3) and 0.5% w/v SDS. Incubate for 30 minutes at 37°C. The extension products are examined on an 8% (endogenous telomerase) or 12% (recombinant telomerase) polyacrylamide-urea gel after DNA is recovered using phenol extraction and ethanol precipitation. A Kodak phosphorimager screen is used to expose dried gels, and the outcomes are examined. | ||
| Cell Assay | In triplicate, the cells are plated in full RPMI 1640 medium containing different concentrations of BIBR1532. Following a 24-to 72-hour period, water-soluble tetrazolium (WST-1) is introduced, and mitochondrial reductase systems convert it into formazan. After two, three, and four hours of incubation, the amount of formazan dye formed increases due to an increase in the number of viable cells, which causes an increase in the activity of mitochondrial dehydrogenases. This formazan dye is then quantified using an ELISA reader. | ||
| Animal Protocol |
|
||
| References |
[1]. Mechanism of human telomerase inhibition by BIBR1532, a synthetic, non-nucleosidic drug candidate. J Biol Chem. 2002 May 3;277(18):15566-72. [2]. Selective cytotoxicity and telomere damage in leukemia cells using the telomerase inhibitor BIBR1532. Blood. 2005 Feb 15;105(4):1742-9. [3]. Pharmacological telomerase inhibition can sensitize drug-resistant and drug-sensitive cells to chemotherapeutic treatment. Mol Pharmacol. 2005 Sep;68(3):779-86. [4]. Short telomeres and high telomerase activity in T-cell prolymphocytic leukemia. Leukemia. 2007 Dec;21(12):2456-62. [5]. Targeted inhibition of telomerase activity combined with chemotherapy demonstrates synergy in eliminating ovarian cancer spheroid-forming cells. Gynecol Oncol. 2012 Mar;124(3):598-605. |
||
| Additional Infomation | 2-[[3-(2-naphthalenyl)-1-oxobut-2-enyl]amino]benzoic acid is an amidobenzoic acid. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (7.54 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (7.54 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 3: 30% PEG400+0.5% Tween80+5% Propylene glycol : 30mg/mL (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0179 mL | 15.0893 mL | 30.1787 mL | |
| 5 mM | 0.6036 mL | 3.0179 mL | 6.0357 mL | |
| 10 mM | 0.3018 mL | 1.5089 mL | 3.0179 mL |