BGP-15 is a potent PARP inhibitor that can protect against heart failure and atrial fibrillation in mice. At 200 μM, BGP-15 inhibits the oxidative damage caused by imatinib mesylate, reduces the loss of high-energy phosphates, modifies the signaling effect of imatinib mesylate by blocking p38 MAP kinase and JNK activation, and stimulates Akt and GSK-3beta phosphorylation. An in-vivo investigation revealed that in two mouse models of HF and AF, BGP-15 enhanced cardiac function and decreased arrhythmic episodes. BGP-15 was linked to higher IGF1R phosphorylation in these models.
Physicochemical Properties
| Molecular Formula | C14H24CL2N4O2 | |
| Molecular Weight | 351.27 | |
| Exact Mass | 278.174 | |
| Elemental Analysis | C, 60.41; H, 7.97; N, 20.13; O, 11.50 | |
| CAS # | 66611-38-9 | |
| Related CAS # |
|
|
| PubChem CID | 9817104 | |
| Appearance | Light yellow to yellow solid powder | |
| LogP | 1.203 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 5 | |
| Rotatable Bond Count | 6 | |
| Heavy Atom Count | 20 | |
| Complexity | 306 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | OC(CN1CCCCC1)CONC(C1=CC=CN=C1)=N |
|
| InChi Key | MVLOQULXIYSERZ-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C14H22N4O2/c15-14(12-5-4-6-16-9-12)17-20-11-13(19)10-18-7-2-1-3-8-18/h4-6,9,13,19H,1-3,7-8,10-11H2,(H2,15,17) | |
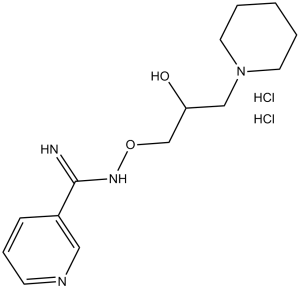
| Chemical Name | N'-(2-hydroxy-3-piperidin-1-ylpropoxy)pyridine-3-carboximidamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | PARP (IC50 =120 μM) | |
| ln Vitro |
|
|
| ln Vivo |
|
|
| Enzyme Assay | The protective effect of O-(3-piperidino-2-hydroxy-1-propyl)nicotinic amidoxime (BGP-15) against ischemia-reperfusion-induced injury was studied in the Langendorff heart perfusion system. To understand the molecular mechanism of the cardioprotection, the effect of BGP-15 on ischemic-reperfusion-induced reactive oxygen species (ROS) formation, lipid peroxidation single-strand DNA break formation, NAD(+) catabolism, and endogenous ADP-ribosylation reactions were investigated. These studies showed that BGP-15 significantly decreased leakage of lactate dehydrogenase, creatine kinase, and aspartate aminotransferase in reperfused hearts, and reduced the rate of NAD(+) catabolism. In addition, BGP-15 dramatically decreased the ischemia-reperfusion-induced self-ADP-ribosylation of nuclear poly(ADP-ribose) polymerase(PARP) and the mono-ADP-ribosylation of an endoplasmic reticulum chaperone GRP78. These data raise the possibility that BGP-15 may have a direct inhibitory effect on PARP. This hypothesis was tested on isolated enzyme, and kinetic analysis showed a mixed-type (noncompetitive) inhibition with a K(i) = 57 +/- 6 microM. Furthermore, BGP-15 decreased levels of ROS, lipid peroxidation, and single-strand DNA breaks in reperfused hearts. These data suggest that PARP may be an important molecular target of BGP-15 and that BGP-15 decreases ROS levels and cell injury during ischemia-reperfusion in the heart by inhibiting PARP activity [4]. | |
| Cell Assay | According to a previous study, BGP-15 at 200 μM could prevent oxidative damages caused by imatinib, reduce the loss of high-energy phosphates, change the way imatinib signals by preventing the activation of JNK and p38 MAP kinase, as well as phosphorylate GSK-3β and Akt. | |
| Animal Protocol |
Male adult HF+AF and Ntg mice, who are approximately 4 months old, are given BGP-15 (15 mg/kg daily in saline) or left untreated (oral gavage with saline or no gavage) for 4 weeks. In the HF+AF model, gavage with saline has no effect on morphological or functional parameters. Thus, mice receiving saline injection and mice left untreated (no gavage) are grouped together and referred to as HF+AF control. ECG and echocardiography scans are carried out both before and after therapy. Experimental protocols[3] Protocol 1: Adult (~4 month) male HF+AF and Ntg mice were administered with BGP-15 (15 mg kg−1 per day in saline, N-Gene Research Laboratories) for 4 weeks by oral gavage or remained untreated (oral gavage with saline or no gavage). Gavage with saline had no effect on morphological or functional parameters in the HF+AF model (Supplementary Fig. 3). Therefore, untreated mice (no gavage) and mice administered saline are combined and referred to as HF+AF control. Echocardiography and ECG studies were performed before and after treatment.[3] Protocol 2: To determine whether BGP-15 provided protection via HSP70, BGP-15 (15 mg kg−1 per day, oral gavage) was administered to adult (~14 weeks) male and female HF+AF mice deficient for HSP70 (HF+AF-HSP70 KO) for 4 weeks.[3] Protocol 3: To assess whether an increase in HSP70 could mediate protection in the HF+AF model, male HF+AF mice overexpressing HSP70 (HF+AF-HSP70 Tg) were generated and characterized at ~12–13 weeks.[3] Protocol 4: To examine whether overexpression of IGF1R in the heart could provide protection in the HF+AF model, male HF+AF mice overexpressing IGF1R (HF+AF-IGF1R Tg) were generated and characterized at ~16–17 weeks.[3] Protocol 5: To determine whether IGF1R could mediate protection in the HF+AF model independent of HSP70, male and female HF+AF-IGF1R Tg-HSP70 KO mice were generated and characterized at ~11 weeks.[3] Protocol 6: To examine whether BGP-15 had the capacity to provide protection in an additional model with HF and AF, 11- to 12-month-old male MURC Tg were administered with BGP-15 (15 mg kg−1 per day, oral gavage) or saline for 4 weeks.[3] To assess whether BGP-15 administration could confer effects on an already established dystrophic pathology, 20-week-old mdx and 8-week-old dko mice were administered BGP-15 (15 mg/kg in 0.9% sterile saline; N-Gene Research Laboratories Inc., New York, NY) daily via oral gavage for 4 (dko) or 5 (mdx) weeks. Age-matched vehicle-treated dystrophic and healthy wild-type control (C57BL/10) mice received an equivalent volume of 0.9% sterile saline via daily oral gavage. Because of the severity of the dko phenotype, a shorter treatment period was used with a significant number of mice reaching humane end point criteria (ie, kyphosis score of 5 and sustained 15% loss of body mass) after 12 weeks of age. The average lifespan of mice in our dko colony was approximately 14 to 15 weeks, with the severity of the dystrophic pathology at 8 weeks of age (when treatment commenced) indicated by an average kyphosis score of 2.5. The kyphosis score indicates the severity of spinal curvature on palpation of conscious mice and ranked 1 to 5, with 1 indicating no spinal deformity and 5 being the most severe. To assess the effect of BGP-15 administration as a preventive treatment for the dystrophic cardiomyopathy and to confirm previous findings on skeletal muscles of young mice,14 4-week-old dko mice were administered BGP-15 (15 mg/kg in 0.9% sterile saline daily via oral gavage) for 5 to 6 weeks, with other groups of aged-matched dko and C57BL/10 mice treated similarly with vehicle only. Because BGP-15 is a hydroxylamine derivative that affects only stressed cells, a group of C57BL/10 mice treated with BGP-15 was not included.10,14,34 Previous studies investigating BGP-15 effects on skeletal muscle and heart observed no morphological or functional changes in either tissue, in wild-type mice after long-term treatment.14,34 To assess Hsp72 induction via BGP-15, 4- and 10-week-old dko mice and age-matched C57BL/10 mice were administered a single bolus of BGP-15 (15 mg/kg) via oral gavage, and the tibialis anterior (TA) muscles, heart, and diaphragm were excised 6 hours later, frozen in liquid nitrogen, and stored at −80°C for later analyses.[1] |
|
| References |
[1]. BGP-15 Improves Aspects of the Dystrophic Pathology in mdx and dko Mice with Differing Efficacies in Heart and Skeletal Muscle. Am J Pathol. 2016 Dec;186(12):3246-3260. [2]. The chaperone co-inducer BGP-15 alleviates ventilation-induced diaphragm dysfunction. Sci Transl Med. 2016 Aug 3;8(350):350ra10. [3]. The small-molecule BGP-15 protects against heart failure and atrial fibrillation in mice. Nat Commun. 2014 Dec 9;5:5705. [4]. Improvement of insulin sensitivity by a novel drug candidate, BGP-15, in different animal studies. Metab Syndr Relat Disord. 2014 Mar;12(2):125-31. [5]. BGP-15, a PARP-inhibitor, prevents imatinib-induced cardiotoxicity by activating Akt and suppressing JNK and p38 MAP kinases. Mol Cell Biochem. 2012 Jun;365(1-2):129-37. [6]. BGP-15, a nicotinic amidoxime derivate protecting heart from ischemia reperfusion injury through modulation of poly(ADP-ribose) polymerase. Biochem Pharmacol. 2000 Apr 15;59(8):937-45. |
|
| Additional Infomation |
Duchenne muscular dystrophy is a severe and progressive striated muscle wasting disorder that leads to premature death from respiratory and/or cardiac failure. We have previously shown that treatment of young dystrophic mdx and dystrophin/utrophin null (dko) mice with BGP-15, a coinducer of heat shock protein 72, ameliorated the dystrophic pathology. We therefore tested the hypothesis that later-stage BGP-15 treatment would similarly benefit older mdx and dko mice when the dystrophic pathology was already well established. Later stage treatment of mdx or dko mice with BGP-15 did not improve maximal force of tibialis anterior (TA) muscles (in situ) or diaphragm muscle strips (in vitro). However, collagen deposition (fibrosis) was reduced in TA muscles of BGP-15-treated dko mice but unchanged in TA muscles of treated mdx mice and diaphragm of treated mdx and dko mice. We also examined whether BGP-15 treatment could ameliorate aspects of the cardiac pathology, and in young dko mice it reduced collagen deposition and improved both membrane integrity and systolic function. These results confirm BGP-15's ability to improve aspects of the dystrophic pathology but with differing efficacies in heart and skeletal muscles at different stages of the disease progression. These findings support a role for BGP-15 among a suite of pharmacological therapies for Duchenne muscular dystrophy and related disorders.[1] Ventilation-induced diaphragm dysfunction (VIDD) is a marked decline in diaphragm function in response to mechanical ventilation, which has negative consequences for individual patients' quality of life and for the health care system, but specific treatment strategies are still lacking. We used an experimental intensive care unit (ICU) model, allowing time-resolved studies of diaphragm structure and function in response to long-term mechanical ventilation and the effects of a pharmacological intervention (the chaperone co-inducer BGP-15). The marked loss of diaphragm muscle fiber function in response to mechanical ventilation was caused by posttranslational modifications (PTMs) of myosin. In a rat model, 10 days of BGP-15 treatment greatly improved diaphragm muscle fiber function (by about 100%), although it did not reverse diaphragm atrophy. The treatment also provided protection from myosin PTMs associated with HSP72 induction and PARP-1 inhibition, resulting in improvement of mitochondrial function and content. Thus, BGP-15 may offer an intervention strategy for reducing VIDD in mechanically ventilated ICU patients.[2] Heart failure (HF) and atrial fibrillation (AF) share common risk factors, frequently coexist and are associated with high mortality. Treatment of HF with AF represents a major unmet need. Here we show that a small molecule, BGP-15, improves cardiac function and reduces arrhythmic episodes in two independent mouse models, which progressively develop HF and AF. In these models, BGP-15 treatment is associated with increased phosphorylation of the insulin-like growth factor 1 receptor (IGF1R), which is depressed in atrial tissue samples from patients with AF. Cardiac-specific IGF1R transgenic overexpression in mice with HF and AF recapitulates the protection observed with BGP-15. We further demonstrate that BGP-15 and IGF1R can provide protection independent of phosphoinositide 3-kinase-Akt and heat-shock protein 70; signalling mediators often defective in the aged and diseased heart. As BGP-15 is safe and well tolerated in humans, this study uncovers a potential therapeutic approach for HF and AF.[3] Background: Insulin resistance has been recognized as the most significant predictor of further development of type 2 diabetes mellitus (T2DM). Here we investigated the effect of a heat shock protein (HSP) co-inducer, BGP-15, on insulin sensitivity in different insulin-resistant animal models and compared its effect with insulin secretagogues and insulin sensitizers. Methods: Insulin sensitivity was assessed by the hyperinsulinemic euglycemic glucose clamp technique in normal and cholesterol-fed rabbits and in healthy Wistar and Goto-Kakizaki (GK) rats in dose-ranging studies. We also examined the effect of BGP-15 on streptozotocin-induced changes in the vasorelaxation of the aorta in Sprague-Dawley rats. Results: BGP-15 doses of 10 and 30 mg/kg increased insulin sensitivity by 50% and 70%, respectively, in cholesterol-fed but not in normal rabbits. After 5 days of treatment with BGP-15, the glucose infusion rate was increased in a dose-dependent manner in genetically insulin-resistant GK rats. The most effective dose was 20 mg/kg, which showed a 71% increase in insulin sensitivity compared to control group. Administration of BGP-15 protected against streptozotocin-induced changes in vasorelaxation, which was similar to the effect of rosiglitazone. Conclusion: Our results indicate that the insulin-sensitizing effect of BGP-15 is comparable to conventional insulin sensitizers. This might be of clinical utility in the treatment of T2DM.[4] |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8468 mL | 14.2341 mL | 28.4681 mL | |
| 5 mM | 0.5694 mL | 2.8468 mL | 5.6936 mL | |
| 10 mM | 0.2847 mL | 1.4234 mL | 2.8468 mL |