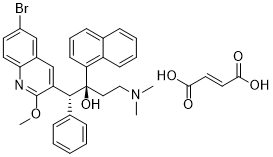
Bedaquiline FUMARATE (formerly also known as TMC207 and R207910; sold under the trade name Sirturo) is the FUMARATE salt of bedaquiline, which is an orally bioavailable and diarylquinoline-based anti-TB (tuberculosis) drug used specifically to treat multi-drug-resistant tuberculosis (MDR-TB) when other treatments cannot be used. It acts by inhibiting mycobacterial ATP synthase. Bedaquiline should be used along with at least three other medications for tuberculosis. Bedaquiline was approved for medical use in the United States in 2012. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. The cost for six months is approximately $900 USD in low income countries, $3,000 USD in middle income countries, and $30,000 USD in high income countries.
Physicochemical Properties
| Molecular Formula | C36H35BRN2O6 |
| Molecular Weight | 671.59 |
| Exact Mass | 670.167 |
| Elemental Analysis | C, 64.38; H, 5.25; Br, 11.90; N, 4.17; O, 14.29 |
| CAS # | 845533-86-0 |
| Related CAS # | Bedaquiline;843663-66-1;(Rac)-Bedaquiline;654655-80-8 |
| PubChem CID | 24812732 |
| Appearance | White to off-white solid powder |
| LogP | 6.842 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 10 |
| Heavy Atom Count | 45 |
| Complexity | 834 |
| Defined Atom Stereocenter Count | 2 |
| SMILES | OC(/C=C/C(O)=O)=O.BrC1=CC=C(N=C(OC)C([C@H]([C@@](C2=CC=CC3=C2C=CC=C3)(O)CCN(C)C)C4=CC=CC=C4)=C5)C5=C1 |
| InChi Key | ZLVSPMRFRHMMOY-WWCCMVHESA-N |
| InChi Code | InChI=1S/C32H31BrN2O2.C4H4O4/c1-35(2)19-18-32(36,28-15-9-13-22-10-7-8-14-26(22)28)30(23-11-5-4-6-12-23)27-21-24-20-25(33)16-17-29(24)34-31(27)37-3;5-3(6)1-2-4(7)8/h4-17,20-21,30,36H,18-19H2,1-3H3;1-2H,(H,5,6)(H,7,8)/b;2-1+/t30-,32-;/m1./s1 |
| Chemical Name | (1R,2S)-1-(6-Bromo-2-methoxy-3-quinolyl)-4-dimethylamino-2-(1-naphthyl)-1-phenyl-butan-2-ol fumarate |
| Synonyms | R207910 fumarate; TMC-207 fumarate; R-207910; TMC 207; R 207910; TMC207 fumarate; Bedaquiline fumarate; trade name: Sirturo |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Mtb F1FO-ATP synthase |
| ln Vitro | TDR M. tuberculosis strains are inhibited in growth by bedaquiline, with MIC values ranging from 0.125 to 0.5 mg/L[1]. With MIC50 and MIC90 values of 0.03 and 16 mg/L, respectively, bedaquiline has the strongest activity against Mycobacterium avium among slowly growing mycobacteria (SGM). Among the mycobacteria that grow quickly (RGM), Mycobacterium abscessus subsp.With MIC50 and MIC90 values of 0.13 and >16 mg/L, respectively, for both species, abscessus (M. abscessus) and Mycobacterium abscessus subsp. massiliense (M. massiliense) appear to be more susceptible to bedaquiline than Mycobacterium fortuitum. Moderate in vitro activity of bedaquiline against NTM species is also demonstrated[2]. In vitro activity of bedaquiline against Mycobacterium tuberculosis, including multidrug-resistant M tuberculosis, is very good[3]. |
| ln Vivo | BDQ was highly efficacious in a zebrafish model of M. abscessus infection. Remarkably, a very short period of treatment was sufficient to protect the infected larvae from M. abscessus-induced killing. This was corroborated with reduced numbers of abscesses and cords, considered to be major pathophysiological signs in infected zebrafish. [7] |
| Enzyme Assay | Intracellular ATP quantification. Intracellular ATP levels were determined using a 96-well flat-bottom plate, as described previously for M. tuberculosis. M. abscessus was exposed to BDQ or amikacin (negative control) and incubated for 180 min at 32°C. Twenty-five microliters of M. abscessus culture was mixed with an equal volume of the BacTiter-Glo reagent in 96-well flat-bottom white plates and incubated for 5 min in the darkness. Luminescence was detected using a BioTek Cytation 3 multimode reader, and the values obtained were plotted using GraphPad Prism 6 software.[7] |
| Cell Assay | Drug susceptibility testing. [7] The CLSI guidelines were followed to determine the MICs based on the broth microdilution method in CaMHB using an inoculum containing 5 × 106 CFU/ml in the exponential-growth phase. Bacteria (100 μl) were seeded in 96-well plates, and 2 μl of drug at its highest concentration was added to the first wells containing double the volume of bacterial suspension (200 μl). Twofold serial dilutions were then carried out, and incubation with drugs was performed at 30°C for 3 to 5 days. MICs were recorded by visual inspection and by absorbance at 560 nm to confirm visual recording. Experiments were done in triplicate on three independent occasions. Time-kill assay.[7] Microtiter plates were set up as for MIC determination. Serial dilutions of the bacterial suspension were plated after 0, 24, 48, 72, and 96 h of exposure to different drug concentrations. CFU were enumerated after 4 days of incubation at 30°C. |
| Animal Protocol | Assessment of BDQ efficacy in infected zebrafish. [7] Rough M. abscessus CIP104536T (ATCC 19977T) carrying pTEC27 (plasmid 30182; Addgene) and expressing the red fluorescent protein tdTomato was prepared and microinjected in zebrafish embryos, according to procedures described earlier. Briefly, mid-log-phase cultures of M. abscessus expressing tdTomato were centrifuged, washed, and resuspended in phosphate-buffered saline (PBS) supplemented with 0.05% Tween 80 (PBS-T). Bacterial suspensions were then homogenized through a 26-gauge needle and sonicated, and the remaining clumps were allowed to settle down for 5 to 10 min. Bacteria were concentrated to an optical density at 600 nm (OD600) of 1 in PBS-T and injected intravenously (≈2 to 5 nl containing 50 to 300 CFU) into the caudal vein in 30-h-postfertilization (hpf) embryos previously dechorionated and anesthetized. To follow infection kinetics and embryo survival, infected larvae were transferred into 24-well plates (2 embryos/well) and incubated at 28.5°C. The CFU numbers in the inoculum were determined by injection of 2 nl of the bacterial suspension in sterile PBS-T and plating on 7H10 with 500 μg/ml hygromycin. |
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Data from two women taking bedaquiline and one of their breastfed infants indicate that exposure of the infant to the drug via breastmilk is substantial, with one infant having a therapeutic serum level. The clinical consequences of this exposure are unknown. The drug could protect the infant from multidrug-resistant tuberculosis, or could result in adverse effects. If bedaquiline is required by the mother, it is not a reason to discontinue breastfeeding. Monitor breastfed infants for adverse reactions, such as inadequate weight gain, liver toxicity, nausea, arthralgia, headache, hemoptysis, and chest pain. ◉ Effects in Breastfed Infants A woman who was co-infected with HIV and rifampin-resistant tuberculosis took bedaquiline (dosage not stated) as part of her antituberculosis regimen, which consisted of pyrazinamide and other unnamed drugs. At the 1-month follow-up, the infant was small and not gaining weight well, but the mother was nauseated from her medication regimen and had also lost weight. Six months later after completion of the mother’s therapy, her infant’s weight was increasing, following the normal trajectory of the growth chart, and reaching her developmental milestones. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| References |
[1]. Bedaquiline susceptibility test for totally drug-resistant tuberculosis Mycobacterium tuberculosis. J Microbiol. 2017 Apr 20. [2]. TBAJ-876 displays Bedaquiline-like mycobactericidal potency without retaining the parental drug's uncoupler activity. Antimicrob Agents Chemother. 2019 Nov 11. [3]. Bedaquiline: a novel diarylquinoline for multidrug-resistant tuberculosis. Ann Pharmacother. 2014 Jan;48(1):107-15. [4]. In Vitro Activity of Bedaquiline against Nontuberculous Mycobacteria in China. Antimicrob Agents Chemother. 2017 Apr 24;61(5). [5]. Ann Pharmacother.2014 Jan;48(1):107-15. [6]. Antimicrob Agents Chemother.2017 Apr 24;61(5). pii: e02627-16. |
| Additional Infomation |
Bedaquiline fumarate is a fumarate salt prepared from equimolar amounts of bedaquiline and fumaric acid. It is used in combination therapy for the treatment of pulmonary multi-drug resistant tuberculosis by inhibition of ATP synthase, an enzyme essential for the replication of the mycobacteria. It has a role as an antitubercular agent and an ATP synthase inhibitor. It contains a bedaquiline(2+). Bedaquiline Fumarate is the fumarate salt form of bedaquiline, an orally bioavailable diarylquinoline antimycobacterial agent, that can be used in the treatment of pulmonary multi-drug resistant tuberculosis (MDR-TB). Upon oral administration, bedaquiline specifically binds to subunit c of Mycobacterium tuberculosis (M. tuberculosis) adenosine 5'-triphosphate (ATP) synthase, thereby preventing ATP synthase activity. This inhibits ATP synthesis in M. tuberculosis, thereby blocking its energy metabolism and killing M. tuberculosis. See also: Bedaquiline (has active moiety). Drug Indication Sirturo is indicated for use as part of an appropriate combination regimen for pulmonary multidrug resistant tuberculosis (MDR TB) in adults and adolescent patients (12 years to less than 18 years of age and weighing at least 30 kg) when an effective treatment regimen cannot otherwise be composed for reasons of resistance or tolerability. Â Consideration should be given to official guidance on the appropriate use of antibacterial agents. Treatment of multi-drug-resistant tuberculosis |
Solubility Data
| Solubility (In Vitro) |
DMSO : ~100 mg/mL ( ~148.9 mM ) Ethanol : ~4 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.75 mg/mL (4.09 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 27.5 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.75 mg/mL (4.09 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 27.5 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.75 mg/mL (4.09 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 27.5 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: ≥ 2.75 mg/mL (4.09 mM) (saturation unknown) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 5: ≥ 2.75 mg/mL (4.09 mM) (saturation unknown) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 6: Solubility in Formulation 1: ≥ 2.8 mg/mL (4.1 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can take 100 μL of 28 mg/mL DMSO stock solution and add to 400 μL of PEG300, mix well (clear solution); Then add 50 μL of Tween 80 to the above solution, mix well (clear solution); Finally, add 450 μL of saline to the above solution, mix well (clear solution). Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.8 mg/mL (4.1 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can take 100 μL of 28 mg/mL DMSO stock solution and add to 900 μL of 20% SBE-β-CD in saline, mix well (clear solution). Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.8 mg/mL (4.1 mM) (saturation unknown) in 10% DMSO + 90% Corn oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can take 100 μL of 28 mg/mL DMSO stock solution and add to 900 μL of corn oil, mix well (clear solution). Solubility in Formulation 4: ≥ 2.8 mg/mL (4.1 mM) (saturation unknown) in 5% DMSO + 40% PEG300 + 5% Tween80 + + 50% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 5: ≥ 2.8 mg/mL (4.1 mM) (saturation unknown) in 5% DMSO + 95% (20% SBE-β-CD in saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.4890 mL | 7.4450 mL | 14.8900 mL | |
| 5 mM | 0.2978 mL | 1.4890 mL | 2.9780 mL | |
| 10 mM | 0.1489 mL | 0.7445 mL | 1.4890 mL |