Physicochemical Properties
| Molecular Formula | C15H20O3 |
| Molecular Weight | 248.3175 |
| Exact Mass | 248.141 |
| CAS # | 73030-71-4 |
| Related CAS # | 73030-71-4 |
| PubChem CID | 155948 |
| Appearance | Off-white to light yellow solid |
| Density | 1.2±0.1 g/cm3 |
| Boiling Point | 424.6±45.0 °C at 760 mmHg |
| Melting Point | 200-201ºC |
| Flash Point | 181.1±21.5 °C |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.558 |
| LogP | 2.36 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Heavy Atom Count | 18 |
| Complexity | 476 |
| Defined Atom Stereocenter Count | 3 |
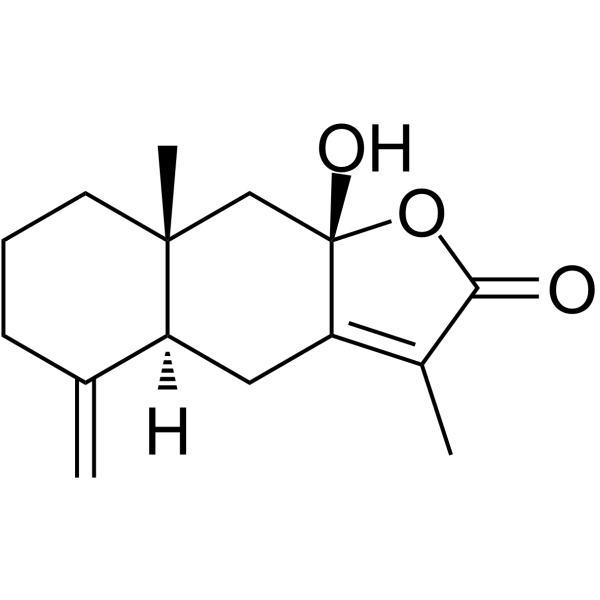
| SMILES | O1C(C(C([H])([H])[H])=C2C([H])([H])[C@@]3([H])C(=C([H])[H])C([H])([H])C([H])([H])C([H])([H])[C@]3(C([H])([H])[H])C([H])([H])[C@]12O[H])=O |
| InChi Key | FBMORZZOJSDNRQ-GLQYFDAESA-N |
| InChi Code | InChI=1S/C15H20O3/c1-9-5-4-6-14(3)8-15(17)12(7-11(9)14)10(2)13(16)18-15/h11,17H,1,4-8H2,2-3H3/t11-,14+,15-/m0/s1 |
| Chemical Name | (4aS,8aR,9aS)-9a-hydroxy-3,8a-dimethyl-5-methylidene-4,4a,6,7,8,9-hexahydrobenzo[f][1]benzofuran-2-one |
| Synonyms | ICodonolactone; Atractylenolide III; 8β-Hydroxyasterolide |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | Atractylenolide III (1-100 μM, 48 h) stimulates A549 cells and induces caspase-3 and caspase-9 activation and PARP localization [1]. Atractylenolide III (1-100 μM, 72 hours) decreases the swelling of HUVECs and Atractylenolide III (1-100 μM) inhibits thymobasalpoietin (TSLP)-induced pro-inflammatory cytokines (IL-6, IL- 1b, TNF-α and IL- ). |
| ln Vivo | Atractylenolide III (5 and 10 mg/kg, p.o.) decreases the 70% ethanol-induced gastrointestinal ulcer in rats and has gastroprotective properties[2]. Atractylenolide III (30 mg/kg, orally administered for 14 or 28 days) attenuates depressive and anxiogenic-like behaviors in rat depression models caused by chronic unpredictable mild stress (CUMS) and lipopolysaccharide (LPS) (HY-D1056)[5]. |
| Cell Assay |
Western Blot Analysis[1] Cell Types: A549 Tested Concentrations: 1, 10, 100 μM Incubation Duration: 24 h, 48 h Experimental Results: Increased active forms of caspase-9, caspase-3 and caspase-3. PARP was cleaved at 48 hrs (hours). The expression of pro-apoptotic protein bax increased at 24 hrs (hours) and induced AIF translocation to the nucleus. |
| Animal Protocol |
Animal/Disease Models: 70% ethanol induced gastric ulcer in rats [2] Doses: 5 and 10 mg/kg Route of Administration: Oral Experimental Results:Inhibition of gastric ulcer Formation and gastric mucosal necrosis and erosion. Downregulates MMP-2/9 expression through activation of TIMP-2 and TIMP-1 expression. |
| References |
[1]. Atractylenolide III, a sesquiterpenoid, induces apoptosis in human lung carcinoma A549 cells via mitochondria-mediated death pathway. Food Chem Toxicol. 2011 Feb;49(2):514-9. [2]. Gastroprotective activity of atractylenolide III from Atractylodes ovata on ethanol-induced gastric ulcer in vitro and in vivo. J Pharm Pharmacol. 2010 Mar;62(3):381-8. [3]. Toxicity of atractylon and atractylenolide III Identified in Atractylodes ovata rhizome to Dermatophagoides farinae and Dermatophagoides pteronyssinus. J Agric Food Chem. 2007 Jul 25;55(15):6027-31. [4]. Ameliorative effect of atractylenolide III in the mast cell proliferation induced by TSLP. Food Chem Toxicol. 2017 Aug;106(Pt A):78-85. [5]. Atractylenolide III reduces depressive- and anxiogenic-like behaviors in rat depression models. Neurosci Lett. 2021 Aug 10;759:136050. |
| Additional Infomation |
Atractylenolide III is a naphthofuran. It has a role as a metabolite. Atractylenolide III has been reported in Codonopsis pilosula, Atractylodes japonica, and other organisms with data available. |
Solubility Data
| Solubility (In Vitro) |
DMSO: ~50 mg/mL (~201.4 mM) Ethanol: ~50 mg/mL (~201.4 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (10.07 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (10.07 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (10.07 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.0271 mL | 20.1353 mL | 40.2706 mL | |
| 5 mM | 0.8054 mL | 4.0271 mL | 8.0541 mL | |
| 10 mM | 0.4027 mL | 2.0135 mL | 4.0271 mL |