Physicochemical Properties
| Molecular Formula | C31H34N2O7 |
| Exact Mass | 546.237 |
| CAS # | 61116-33-4 |
| PubChem CID | 6441014 |
| Appearance | Light yellow to yellow solid powder |
| LogP | 4.015 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 10 |
| Heavy Atom Count | 40 |
| Complexity | 1270 |
| Defined Atom Stereocenter Count | 3 |
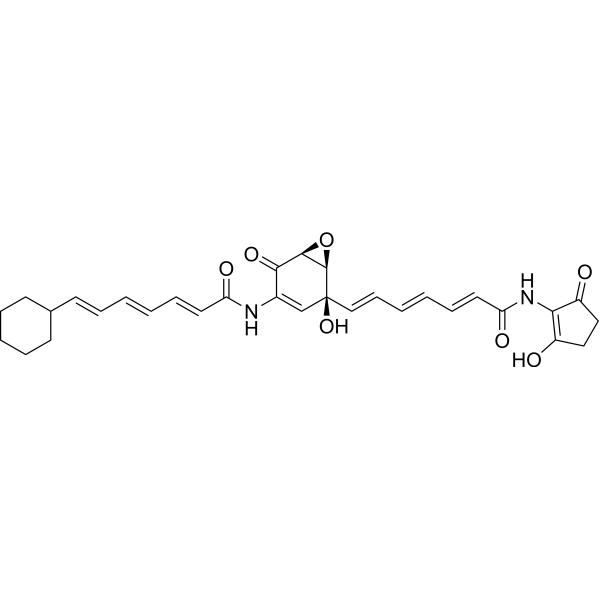
| SMILES | C1CCC(CC1)/C=C/C=C/C=C/C(=O)NC2=C[C@]([C@H]3[C@@H](C2=O)O3)(/C=C/C=C/C=C/C(=O)NC4=C(CCC4=O)O)O |
| InChi Key | SSHVAUUEPNULMP-JHWDTTIQSA-N |
| InChi Code | InChI=1S/C31H34N2O7/c34-23-17-18-24(35)27(23)33-26(37)16-10-3-4-11-19-31(39)20-22(28(38)29-30(31)40-29)32-25(36)15-9-2-1-6-12-21-13-7-5-8-14-21/h1-4,6,9-12,15-16,19-21,29-30,34,39H,5,7-8,13-14,17-18H2,(H,32,36)(H,33,37)/b2-1+,4-3+,12-6+,15-9+,16-10+,19-11+/t29-,30-,31+/m1/s1 |
| Chemical Name | (2E,4E,6E)-7-cyclohexyl-N-[(1S,5S,6R)-5-hydroxy-5-[(1E,3E,5E)-7-[(2-hydroxy-5-oxocyclopenten-1-yl)amino]-7-oxohepta-1,3,5-trienyl]-2-oxo-7-oxabicyclo[4.1.0]hept-3-en-3-yl]hepta-2,4,6-trienamide |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | Asukamycin (1-50 μM; 24 h) has IC50 values of 1-5 μM and decreases the survival of five distinct human cell lines in a concentration-dependent manner[1]. Asukamycin (50 μM; 24 and 72 h; THP-1 cells) significantly increases the activity of intracellular caspase 3 and caspase 8. Asukamycin has antibacterial action against bacteria (18–72 hours) and fungi (0–100 mcg/mL). Between 0.78 and 12.5 mcg/mL, asukamycin reduces the growth of Gram-positive bacteria, and between 25 mcg/mL and Trichophyton mentagrophytes, it inhibits both types of bacteria's growth. |
| ln Vivo | In NZW rabbits, astodrimer (3%, 8 times daily for 4 days, then 4 times daily for 6 days) possesses anti-adenoviral action and is not harmful to rabbit eyes[2]. In the highly vulnerable K18-hACE2 animal model, astodrimer (1% intranasal injection; once daily for 5 days) lessens the severity of SARS-CoV-2 proliferation and pathogenesis[3]. |
| Cell Assay |
Cell Viability Assay[1] Cell Types: Human myeloid THP-1, HL-60, U-118 MG, U-87 MG and U-937 cells Tested Concentrations: 1-50 μM Incubation Duration: 24 hrs (hours) Experimental Results: decreased viability of five different cell lines in a concentration-dependent manner. |
| Animal Protocol |
Animal/Disease Models: Mice[2] Doses: 0-450 mg/kg Route of Administration: intraperitoneal (ip)injection Experimental Results: Had no effect on mice when administered by 450 mg/kg and the acute toxicity (LD50) was 48.5 mg/kg by intraperitoneal (ip)injection. |
| References |
[1]. Antitumor activity of asukamycin, a secondary metabolite from the actinomycete bacterium Streptomyces nodosus subspecies asukaensis. Int J Mol Med. 2009 Nov;24(5):711-5. [2]. A new antibiotic,, asukamycin, produced by Streptomyces. J Antibiot (Tokyo). 1976 Sep;29(9):876-81. |
| Additional Infomation |
Asukamycin is a polyketide that is a member of the manumycin family of antibiotics and exhibits strong antibacterial, antifungal, and antineoplastic activities. Isolated from from the actinomycete bacterium Streptomyces nodosus subsp. asukaensis. It has a role as an antibacterial agent, an antifungal agent, an antimicrobial agent, an antineoplastic agent and a bacterial metabolite. It is an enamide, an epoxide, an organic heterobicyclic compound, a polyketide, a tertiary alcohol and a secondary carboxamide. It is functionally related to a 4-hydroxyprotoasukamycin. Asukamycin has been reported in Streptomyces, Streptomyces nodosus, and Apis cerana with data available. See also: Manumycin F (annotation moved to). |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |