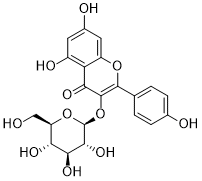
Astragalin is a novel and potent flavonoid. Astragalin inhibits cancer cell growth and migration and triggers apoptosis. The heart and nerves can be protected by astragalin when taken orally.
Physicochemical Properties
| Molecular Formula | C21H20O11 |
| Molecular Weight | 448.38 |
| Exact Mass | 448.1 |
| Elemental Analysis | C, 56.25; H, 4.50; O, 39.25 |
| CAS # | 480-10-4 |
| Related CAS # | 480-10-4 |
| PubChem CID | 5282102 |
| Appearance | Light yellow to yellow solid powder |
| Density | 1.8±0.1 g/cm3 |
| Boiling Point | 823.2±65.0 °C at 760 mmHg |
| Melting Point | 223-229ºC |
| Flash Point | 291.6±27.8 °C |
| Vapour Pressure | 0.0±3.1 mmHg at 25°C |
| Index of Refraction | 1.774 |
| LogP | 1.95 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 4 |
| Heavy Atom Count | 32 |
| Complexity | 719 |
| Defined Atom Stereocenter Count | 5 |
| SMILES | O1[C@]([H])([C@@]([H])([C@]([H])([C@@]([H])([C@@]1([H])C([H])([H])O[H])O[H])O[H])O[H])OC1C(C2=C(C([H])=C(C([H])=C2OC=1C1C([H])=C([H])C(=C([H])C=1[H])O[H])O[H])O[H])=O |
| InChi Key | JPUKWEQWGBDDQB-QSOFNFLRSA-N |
| InChi Code | InChI=1S/C21H20O11/c22-7-13-15(26)17(28)18(29)21(31-13)32-20-16(27)14-11(25)5-10(24)6-12(14)30-19(20)8-1-3-9(23)4-2-8/h1-6,13,15,17-18,21-26,28-29H,7H2/t13-,15-,17+,18-,21+/m1/s1 |
| Chemical Name | 5,7-dihydroxy-2-(4-hydroxyphenyl)-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one |
| Synonyms | Astragalin; K5; Astragaline; Kaempferol-3-beta-glucopyranoside; Kaempferol-3-O-glucoside; Kaempferol-3-glucoside; Kaempferol-3-beta-monoglucoside; UNII-APM8UQ3Z9O; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | p65 |
| ln Vitro |
Astragalin (0-80 μg/mL; 24 h, 48 h, 72 h) does not exhibit any cytotoxicity to normal human colon epithelial cell lines NCM460, but it inhibits cancer cells' viability and migration[1]. Astragalin (80 μg/mL; 4 h, 8 h) disrupts the NF-κB signaling pathway and inhibits NF-κB P65 transcriptional activity in HCT116 cells stimulated by TNF-α[1]. stragalin (20, 40, 80 μg/mL; 48 h) induces cell death in HCT116 cells and halts the cell cycle at the G0/G1 phase[1]. In HCT116, astragalin (20, 40, 80 μg/mL; 48 h) increases the level of apoptin and decreases the level of anti-apoptotic proteins in a dose-dependent manner[1]. |
| ln Vivo | stragalin (25, 50, and 75 mg/kg; p.o.; once every two days; 25 d) decreases tumor volumes, tumor weight, and tumor formation rates as well as p-NFκB and p-iκκα expression in nude mouse model with human colon cancer[1]. |
| References |
[1]. Astragalin Inhibits the Proliferation and Migration of Human Colon Cancer HCT116 Cells by Regulating the NF-κB Signaling Pathway. Front Pharmacol. 2021 Apr 19;12:639256. |
| Additional Infomation |
Kaempferol 3-O-beta-D-glucoside is a kaempferol O-glucoside in which a glucosyl residue is attached at position 3 of kaempferol via a beta-glycosidic linkage. It has a role as a trypanocidal drug and a plant metabolite. It is a kaempferol O-glucoside, a monosaccharide derivative, a trihydroxyflavone and a beta-D-glucoside. It is a conjugate acid of a kaempferol 3-O-beta-D-glucoside(1-). Astragalin has been reported in Persicaria muricata, Camellia sinensis, and other organisms with data available. See also: Moringa oleifera leaf (has part). |
Solubility Data
| Solubility (In Vitro) | DMSO: ~90 mg/mL (~200.7 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (5.58 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (5.58 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (5.58 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 10 mg/mL (22.30 mM) in 1% CMC-Na/saline water (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2303 mL | 11.1513 mL | 22.3025 mL | |
| 5 mM | 0.4461 mL | 2.2303 mL | 4.4605 mL | |
| 10 mM | 0.2230 mL | 1.1151 mL | 2.2303 mL |