Physicochemical Properties
| Molecular Formula | C17H19F3N2O |
| Molecular Weight | 324.3408 |
| Exact Mass | 324.144 |
| Elemental Analysis | C, 62.95; H, 5.90; F, 17.57; N, 8.64; O, 4.93 |
| CAS # | 1338780-86-1 |
| Related CAS # | 941678-49-5; |
| PubChem CID | 54752297 |
| Appearance | Solid powder |
| LogP | 3.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Heavy Atom Count | 23 |
| Complexity | 445 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | FRRHMLGKNPFRKT-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C17H19F3N2O/c18-12-1-2-13(15(20)14(12)19)21-17(23)22-16-10-4-8-3-9(6-10)7-11(16)5-8/h1-2,8-11,16H,3-7H2,(H2,21,22,23) |
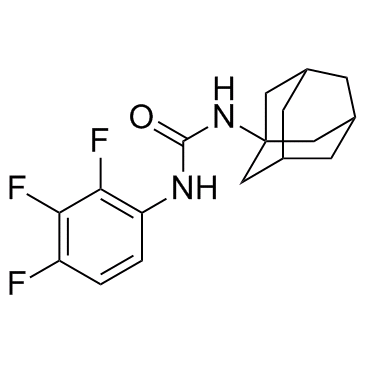
| Chemical Name | 1-(2-Adamantyl)-3-(2,3,4-trifluorophenyl)urea |
| Synonyms | AU1235; AU-1235; AU1235; 1338780-86-1; 1-(1-adamantyl)-3-(2,3,4-trifluorophenyl)urea; CHEMBL1818385; SCHEMBL18423981; SCHEMBL18464994; SCHEMBL18464996; AU 1235 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | MmpL3 |
| ln Vitro | Similar efficaciousness against MDR isolates of M is exhibited by AU1235 in conjunction to fluoroquinolones, ethambutol, and/or streptomycin. resistance to pyrazinamide, rifampicin, and isoniazid in tuberculosis patients. medicinal qualities. Although the minimum inhibitory concentration (MIC) of AU1235 (3.2 to 6.4 μg/ml) is considerably higher than that of Mycobacterium tuberculosis and Mycobacterium bovis BCG, it still inhibits Mycobacterium smegmatis and Mycobacterium fortuitum [2]. |
| Enzyme Assay |
Selection of spontaneous AU1235-resistant mutants of M. tb [2] AU1235-resistant mutants were selected at 37°C (M. tb H37Rv) or 30°C (M. tb H37Ra) on 7H11 plates supplemented with OADC and 0.2 to 0.4 μg ml−1 of the inhibitor (2 to 4 × MIC) and scored for resistance 3 to 7 weeks post-inoculation. Mycolyltransferase assays [2] The TMM transesterification assay described in ref. 24 was used to measure the mycolyltransferase activity of the purified FbpA FbpB and FbpC proteins in the presence of cold TMM purified from M. tb, [U-14C]trehalose and different concentrations of AU1235 (see Supplementary Fig. 5). Purified FbpA and FbpB proteins were obtained through the NIH - TB Vaccine Testing and Research Materials Contract and NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: Ag85A, purified native protein from M. tb H37Rv (NR-14856); Ag85B, purified native protein from M. tb H37Rv (NR-14857). Purified recombinant FbpC (Ag85C) was kindly provided by Dr. K. Dobos. |
| Cell Assay |
Determination of MIC.[1] MICs were determined on either solid or liquid media as described (31, 32). For solid medium, MICs were determined in 24-well plates inoculated with 5 μl of culture at 1 × 105 CFU/ml. Plates were incubated at 37°C for 4 weeks, and the MIC was defined as the minimum concentration that prevented growth. For liquid medium, assays were performed in 96-well plates inoculated with 35 μl of culture at an optical density at 590 nm (OD590) of 0.06 to 0.10; growth was measured by OD590 after 5 days at 37°C. The MIC90 (IC90) was defined as the concentration at which 90% of growth was inhibited. All compounds were dissolved in dimethyl sulfoxide (DMSO). SQ109 and AU1235 were synthesized according to published protocols (4). MIC determinations were performed in at least biological duplicates. Drug susceptibility testing[2] MIC values of various antibiotics against Mycobacterium clinical isolates and recombinant strains were determined in 7H9-OADC-Tween 80 or 7H9-S-OADC-Tween 80 broth at 37°C in 96-well microtiter plates using the colorimetric resazurin microtiter assay and by visual readout for growth. Low-oxygen tension experiments were performed using the Rapid Anaerobic Dormancy (RAD) model as described. Control tubes contained methylene blue dye (1.5 μg ml−1) as an indicator of oxygen depletion. Different concentrations of AU1235 and control drugs (isoniazid, rifampicin, ethambutol and metronidazole) were injected through the septa of oxygen-depleted cultures and the cultures allowed to grow at 37o C with stirring for another 4 days, at which point the septa were removed and cultures serially diluted in saline and plated onto 7H11-OADC agar plates for enumeration of CFU. |
| References |
[1]. McNeil MB, et al. Multiple Mutations in Mycobacterium tuberculosis MmpL3 Increase Resistance to MmpL3 Inhibitors. mSphere. 2020;5(5):e00985-20. Published 2020 Oct 14. [2]. Grzegorzewicz AE, et al. Inhibition of mycolic acid transport across the Mycobacterium tuberculosis plasma membrane. Nat Chem Biol. 2012;8(4):334-341. Published 2012 Feb 19. |
| Additional Infomation |
The Mycobacterium tuberculosis protein MmpL3 performs an essential role in cell wall synthesis, since it effects the transport of trehalose monomycolates across the inner membrane. Numerous structurally diverse pharmacophores have been identified as inhibitors of MmpL3 largely based on the identification of resistant isolates with mutations in MmpL3. For some compounds, it is possible there are different primary or secondary targets. Here, we have investigated resistance to the spiral amine class of compounds. Isolation and sequencing of resistant mutants demonstrated that all had mutations in MmpL3. We hypothesized that if additional targets of this pharmacophore existed, then successive rounds to generate resistant isolates might reveal mutations in other loci. Since compounds were still active against resistant isolates, albeit with reduced potency, we isolated resistant mutants in this background at higher concentrations. After a second round of isolation with the spiral amine, we found additional mutations in MmpL3. To increase our chance of finding alternative targets, we ran a third round of isolation using a different molecule scaffold (AU1235, an adamantyl urea). Surprisingly, we obtained further mutations in MmpL3. Multiple mutations in MmpL3 increased the level and spectrum of resistance to different pharmacophores but did not incur a fitness cost in vitro These results support the hypothesis that MmpL3 is the primary mechanism of resistance and likely target for these pharmacophores.IMPORTANCEMycobacterium tuberculosis is a major global human pathogen, and new drugs and new drug targets are urgently required. Cell wall biosynthesis is a major target of current tuberculosis drugs and of new agents under development. Several new classes of molecules appear to have the same target, MmpL3, which is involved in the export and synthesis of the mycobacterial cell wall. However, there is still debate over whether MmpL3 is the primary or only target for these classes. We wanted to confirm the mechanism of resistance for one series. We identified mutations in MmpL3 which led to resistance to the spiral amine series. High-level resistance to these compounds and two other series was conferred by multiple mutations in the same protein (MmpL3). These mutations did not reduce growth rate in culture. These results support the hypothesis that MmpL3 is the primary mechanism of resistance and likely target for these pharmacophores.[1] Additional mutations in MmpL3 increase the spectrum of resistance. In our previous two rounds of resistant mutant isolation, we did not find any resistant strains with mutations outside MmpL3. Since the strains with two mutations now had relatively high-level resistance to both of our original compounds (>50 μM) (Tables 1 and 2), we were unable to attempt further rounds of resistant strain isolation to IDR-0334448 or IDR-0033216. However, the spectrum of cross-resistance to SQ109 and AU1235 was variable (Table 2), as might be expected if compounds have unique interactions with MmpL3. Therefore, we made use of the observation that the strains were not fully resistant to these compounds and conducted a third round of resistant mutant isolation. We selected two strains, which demonstrated different levels of resistance to SQ109 and no significant resistance to AU1235: (i) LP-0334448-RM102 with F255L and L567P showed a 5.5-fold resistance to SQ109, and (ii) LP-0334448-RM107 with F255L and V646M showed a 3.3-fold increase in resistance to SQ109. These mutations were chosen as they provide resistance against other pharmacophores and are functionally important residues (5, 24, 25). Since the MICs of AU1235 were <2-fold changed, we were able to use this compound to isolate resistant mutants at 5× MIC on solid medium as before. Again, we hypothesized that if additional targets were mutated, then these isolated resistant mutants would not contain mutations in MmpL3. Instead, sequencing of nine strains (three from the LP-0334448-RM102 strain and six from the LP-0334448-RM107 strain) demonstrated that all had additional mutations at F644, either F644L or F644I (Table 3) and had high-level resistance to AU1235 (16-fold and 100-fold shifts, respectively). These strains were also cross resistant to SQ109, albeit at a lower level (5-fold and 6.7-fold, respectively). F644L did not result in increased resistance to a structurally related spiral amine (i.e., IDR-0541243), while F644I conferred a 7-fold shift to resistance (Table 3). This is consistent with the predicted functional importance of F644 and the targeting of this site by multiple pharmacophores.[1] Altogether, the unchanged accumulation of AU1235 in the mmpL3-G253E-expressing M. tb cells and the fact that decreased mmpL3 expression mimicked the phenotypic effects of treating mycobacteria with AU1235 refute the hypothesis that MmpL3 serves as an efflux pump for the adamantyl urea and, instead, confirms this transporter as the direct target of our prototype inhibitor. [2] |
Solubility Data
| Solubility (In Vitro) |
Ethanol : ~65 mg/mL DMSO : 10~25 mg/mL ( 30.83~77.08 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (7.71 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (7.71 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 3: 10% DMSO+90% (20% SBE-β-CD in Saline): ≥ 2.5 mg/mL (7.71 mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0832 mL | 15.4159 mL | 30.8318 mL | |
| 5 mM | 0.6166 mL | 3.0832 mL | 6.1664 mL | |
| 10 mM | 0.3083 mL | 1.5416 mL | 3.0832 mL |