Physicochemical Properties
| Molecular Formula | C25H25F2N7O |
| Molecular Weight | 477.509111166 |
| Exact Mass | 477.208 |
| CAS # | 909561-15-5 |
| PubChem CID | 44208030 |
| Appearance | Brown to black solid powder |
| LogP | 3.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 7 |
| Heavy Atom Count | 35 |
| Complexity | 691 |
| Defined Atom Stereocenter Count | 0 |
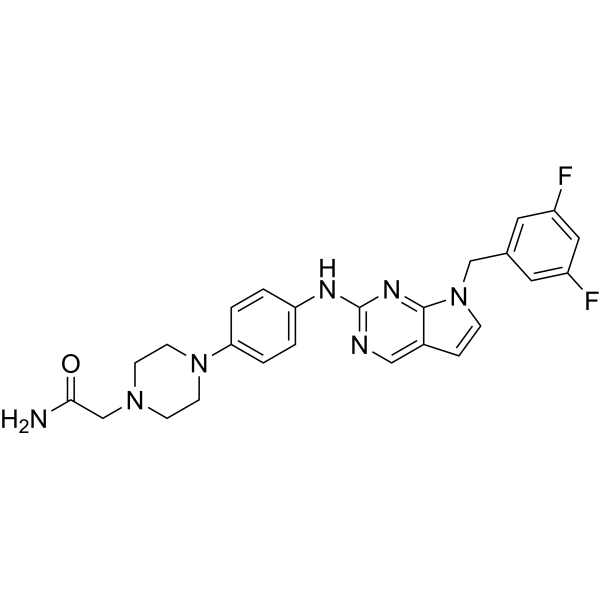
| SMILES | FC1C=C(C=C(C=1)CN1C=CC2=CN=C(N=C12)NC1C=CC(=CC=1)N1CCN(CC(N)=O)CC1)F |
| InChi Key | IJFRMEXSGYTWGY-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C25H25F2N7O/c26-19-11-17(12-20(27)13-19)15-34-6-5-18-14-29-25(31-24(18)34)30-21-1-3-22(4-2-21)33-9-7-32(8-10-33)16-23(28)35/h1-6,11-14H,7-10,15-16H2,(H2,28,35)(H,29,30,31) |
| Chemical Name | 2-[4-[4-[[7-[(3,5-difluorophenyl)methyl]pyrrolo[2,3-d]pyrimidin-2-yl]amino]phenyl]piperazin-1-yl]acetamide |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | STAT6 1.9 nM (IC50) CYP3A4 |
| ln Vitro | AS1810722 (compound 24) has no effect on the production of IFN-γ but suppresses the synthesis of IL-4 with an IC50 of 2.4 nM [1]. |
| ln Vivo | In an antigen-induced mouse asthma paradigm called Infiltration, AS1810722 (Compound 24; 0.03-0.3 mg/kg; oral; 30 minutes before OVA exposure, 24 hours, and 48 hours after OVA exposure) suppresses pulmonary eosinophils in a dose-dependent manner[1]. In an antigen-induced mouse asthma model, AS1810722 suppresses Th2 differentiation in vitro with an IC50 of 2.4 nM following oral administration; it has no effect on the differentiation of type 1 helper T (Th1) cells or eosinophil infiltration[1]. |
| Animal Protocol |
Animal/Disease Models: Female balb/c (Bagg ALBino) mouse asthmatic model by intraperitoneal (ip) injection of ovalbumin (OVA)-containing aluminum hydroxide gel[1] Doses: 0.03-0.3 mg/kg Route of Administration: po (oral gavage) 30 min before, and 24 and 48 h after OVA exposure Experimental Results: Suppressed eosinophil infiltration in the lung in a dose-dependent manner. |
| References |
[1]. Novel 7H-pyrrolo[2,3-d]pyrimidine derivatives as potent and orally active STAT6 inhibitors. Bioorg Med Chem. 2009 Oct 1;17(19):6926-36. |
Solubility Data
| Solubility (In Vitro) | DMSO: 62.5 mg/mL (130.89 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: 2.08 mg/mL (4.36 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0942 mL | 10.4710 mL | 20.9420 mL | |
| 5 mM | 0.4188 mL | 2.0942 mL | 4.1884 mL | |
| 10 mM | 0.2094 mL | 1.0471 mL | 2.0942 mL |