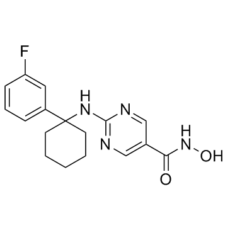
ACY-775, a novel pyrimidine hydroxyl amide small molecule, is a brain bioavailable, highly potent and isoform-selective inhibitor of HDAC6 with IC50 of 7.5 nM. ACY-738 and ACY-775 exhibit a selectivity of 60–1500 times greater than class I HDACs while inhibiting HDAC6 with low nanomolar potency. When it comes to stimulating mouse exploratory behaviors in unfamiliar but novel environments, ACY-738 and ACY-775 cause dramatic increases in α-tubulin acetylation in the brain, unlike tubastatin A, a reference HDAC6 inhibitor with similar potency and peripheral activity but more limited brain bioavailability. Remarkably, ACY-738 and ACY-775 exhibit antidepressant-like effects in the tail suspension test and social defeat paradigm, similar to other HDAC inhibitors like SAHA and MS-275, despite having no discernible effect on histone acetylation. The central inhibition of HDAC6 is directly responsible for the effects of ACY-738 and ACY-775, as these effects are completely abolished in mice that have a loss of function of HDAC6 that is specific to the neurons. Moreover, a behaviorally inactive dosage of ACY-738 combined with a subactive dose of the selective serotonin reuptake inhibitor citalopram significantly enhances the anti-immobility effect. These findings support the viability of this HDAC isoform as a possible target for the development of antidepressants and validate novel isoform-selective probes for in vivo pharmacological investigations of HDAC6 in the CNS.
Physicochemical Properties
| Molecular Formula | C17H19FN4O2 | |
| Molecular Weight | 330.36 | |
| Exact Mass | 330.15 | |
| Elemental Analysis | C, 61.81; H, 5.80; F, 5.75; N, 16.96; O, 9.69 | |
| CAS # | 1375466-18-4 | |
| Related CAS # |
|
|
| PubChem CID | 57382288 | |
| Appearance | White to off-white solid powder | |
| Density | 1.1±0.1 g/cm3 | |
| Boiling Point | 589.7±45.0 °C at 760 mmHg | |
| Flash Point | 310.4±28.7 °C | |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C | |
| Index of Refraction | 1.566 | |
| LogP | 1.54 | |
| Hydrogen Bond Donor Count | 3 | |
| Hydrogen Bond Acceptor Count | 6 | |
| Rotatable Bond Count | 4 | |
| Heavy Atom Count | 24 | |
| Complexity | 423 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | FC1=CC=CC(=C1)C1(CCCCC1)NC1N=CC(C(NO)=O)=CN=1 |
|
| InChi Key | IYBURCQQEUNLDL-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C17H19FN4O2/c18-14-6-4-5-13(9-14)17(7-2-1-3-8-17)21-16-19-10-12(11-20-16)15(23)22-24/h4-6,9-11,24H,1-3,7-8H2,(H,22,23)(H,19,20,21) | |
| Chemical Name | 2-[[1-(3-fluorophenyl)cyclohexyl]amino]-N-hydroxypyrimidine-5-carboxamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | HDAC6 (IC50 = 7.5 nM); HDAC1 (IC50 = 2123 nM); HDAC2 (IC50 = 2570 nM); HDAC3 (IC50 = 11223 nM) | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Cell Assay | The line of immortalized rat raphe neuronal precursors, known as undifferentiated RN46A-B14 cells, is cultured. For four hours, they are given either 2.5 μM ACY-738, ACY-775, tubastatin A, 0.6 μM TSA, or 0.1% DMSO as a vehicle. Samples are prepared with a histone extraction kit, and protein assay is used to quantify the results. | ||
| Animal Protocol |
|
||
| References |
[1]. Development of Improved HDAC6 Inhibitors as Pharmacological Therapy for Axonal Charcot-Marie-Tooth Disease. Neurotherapeutics. 2017 Apr; 14(2): 417-428. [2]. Antidepressant-Like Properties of Novel HDAC6-Selective Inhibitors with Improved Brain Bioavailability. Neuropsychopharmacology. 2014 Jan; 39(2): 389-400. [3]. Target deconvolution of HDAC pharmacopoeia reveals MBLAC2 as common off-target. Nat Chem Biol. 2022 Apr 28. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (7.57 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (7.57 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (7.57 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0270 mL | 15.1350 mL | 30.2700 mL | |
| 5 mM | 0.6054 mL | 3.0270 mL | 6.0540 mL | |
| 10 mM | 0.3027 mL | 1.5135 mL | 3.0270 mL |