A-1331852 (A1331852) is a potent and selective BCL-XL inhibitor with anticancer and immunomodulatory effects. Treatment for autoimmune, immune, and cancerous diseases may benefit from it. In comparison to navitoclax, a previously reported BCL-XL inhibitor, and both of its analogs, A-1155463 and A-1331852, respectively, these compounds showed cellular activity that was 10- to 50-fold more potent. Additionally, with median IC50 values in the low nanomolar range, A-1331852 could specifically disassemble BCL-XL-BIM complexes and trigger the hallmarks of apoptosis in BCL-dependent Molt-4 cells, but it had no effect on MEF cells lacking BAK or BAX.
Physicochemical Properties
| Molecular Formula | C38H38N6O3S | |
| Molecular Weight | 658.81 | |
| Exact Mass | 658.272 | |
| Elemental Analysis | C, 69.28; H, 5.81; N, 12.76; O, 7.29; S, 4.87 | |
| CAS # | 1430844-80-6 | |
| Related CAS # |
|
|
| PubChem CID | 71565985 | |
| Appearance | White to light yellow solid powder | |
| Density | 1.5±0.1 g/cm3 | |
| Index of Refraction | 1.792 | |
| LogP | 6.67 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 8 | |
| Rotatable Bond Count | 7 | |
| Heavy Atom Count | 48 | |
| Complexity | 1180 | |
| Defined Atom Stereocenter Count | 0 | |
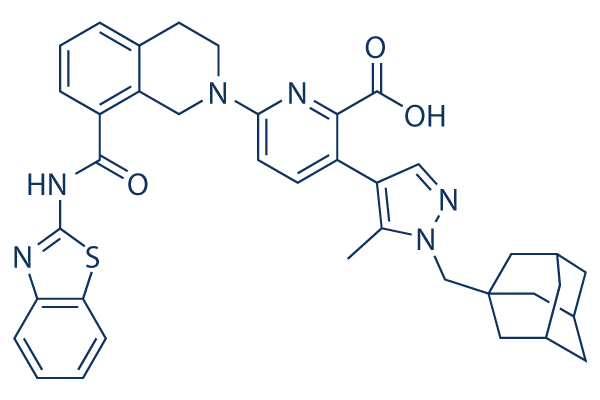
| SMILES | S1C2=CC=CC=C2N=C1NC(C1=CC=CC2=C1CN(CC2)C1C=CC(=C(C(=O)O)N=1)C1C=NN(C=1C)CC12CC3CC(CC(C3)C1)C2)=O |
|
| InChi Key | QCQQONWEDCOTBV-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C38H38N6O3S/c1-22-29(19-39-44(22)21-38-16-23-13-24(17-38)15-25(14-23)18-38)27-9-10-33(41-34(27)36(46)47)43-12-11-26-5-4-6-28(30(26)20-43)35(45)42-37-40-31-7-2-3-8-32(31)48-37/h2-10,19,23-25H,11-18,20-21H2,1H3,(H,46,47)(H,40,42,45) | |
| Chemical Name | 3-[1-(1-adamantylmethyl)-5-methylpyrazol-4-yl]-6-[8-(1,3-benzothiazol-2-ylcarbamoyl)-3,4-dihydro-1H-isoquinolin-2-yl]pyridine-2-carboxylic acid | |
| Synonyms | A1331852; A-1331852; 3-[1-(1-adamantylmethyl)-5-methylpyrazol-4-yl]-6-[8-(1,3-benzothiazol-2-ylcarbamoyl)-3,4-dihydro-1H-isoquinolin-2-yl]pyridine-2-carboxylic acid; CHEMBL3793424; 3-(1-(((3r,5r,7r)-adamantan-1-yl)methyl)-5-methyl-1H-pyrazol-4-yl)-6-(8-(benzo[d]thiazol-2-ylcarbamoyl)-3,4-dihydroisoquinolin-2(1H)-yl)picolinic acid; A1331852; A 1331852 | |
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Bcl-xL (Ki = 0.01 nM); Bcl-W (Ki = 4 nM); Bcl-2 (Ki = 6 nM); Mcl-1 (Ki = 142 nM) |
| ln Vitro |
A-1331852 exhibits remarkable potency both as a single agent and in combination with TKIs in killing primary CD34+ CML cell. Additionally, it has a remarkable capacity to induce apoptosis in these cells as early as 1 hour after treatment at low nanomolar concentrations[2]. In this study, researchers describe the discovery of A-1331852, a first-in-class orally active BCL-XL inhibitor that selectively and potently induces apoptosis in BCL-XL-dependent tumor cells. This molecule was generated by re-engineering our previously reported BCL-XL inhibitor A-1155463 using structure-based drug design. Key design elements included rigidification of the A-1155463 pharmacophore and introduction of sp3-rich moieties capable of generating highly productive interactions within the key P4 pocket of BCL-XL. A-1331852 has since been used as a critical tool molecule for further exploring BCL-2 family protein biology, while also representing an attractive entry into a drug discovery program. The cell-killing efficacy of A-1331852 (13) against MOLT-4 cells was improved by 10- to 30-fold relative to the cyclohexane 12, while maintaining selectivity against the RS4;11 cell line. Thus, A-1331852 (13) exhibited a 6 nM EC50 against the MOLT-4 cell line, a level of in vitro efficacy 20-fold more potent than our previously disclosed tool compound A-1155463 (1).Reference: ACS Med Chem Lett. 2020 Oct 8; 11(10): 1829–1836. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7549103/ |
| ln Vivo |
As a single agent, A-1331852 induces tumor regressions in the Molt-4 xenograft model, demonstrating antitumor efficacy[1]. Orally bioavailable BCL-XL–selective inhibitor A-1331852 enhances the efficacy of docetaxel in vivo[1] Researchers assessed the ability of a selective BCL-XL inhibitor to enhance the efficacy of docetaxel in vivo. To this end, we used structure-based design to generate A-1331852 (Fig. 4A), a BCL-XL–selective inhibitor with oral bioavailability. A-1331852 is a potent BCL-XL inhibitor, binding BCL-XL with a Ki value of <0.010 nM and demonstrating cellular activity 10- to 50-fold more potent than A-1155463 and navitoclax, respectively (Table 1). This molecule selectively disrupts BCL-XL–BIM complexes and induces the hallmarks of apoptosis in BCL-XL–dependent Molt-4 cells with median inhibitory concentration (IC50) values in the low nanomolar range (Fig. 4, B to E, and Table 1) but does not affect MEF cells lacking BAK or BAX (fig. S5). Moreover, A-1331852 demonstrates antitumor efficacy in the Molt-4 xenograft model, inducing tumor regressions as a single agent (Fig. 4F). Additionally, A-1331852 combines with venetoclax to recapitulate the efficacy of navitoclax in the NCI-H1963.FP5 xenograft model of SCLC (Fig. 4G), thus providing in vivo confirmation of the combination studies shown in Fig. 2 (B and D).[1] Inhibition of tumor growth by A-1331852 combined with docetaxel was determined in seven subcutaneous xenograft models of solid tumors, including breast cancer, NSCLC, and ovarian cancer. Given as a single agent, A-1331852 significantly (P < 0.05) inhibited tumor growth in all seven models (Table 4). Although its single-agent activity was modest (TGImax < 60% in five of seven models), A-1331852 increased the efficacy of docetaxel in all seven models. As shown in Table 4, the maximum tumor growth inhibition (TGImax) for A-1331852 as a single agent ranged between 34% (OVCAR-5) and 67% (A549-FP3). The most durable response to A-1331852 was a tumor growth delay (TGD) of 108%, observed in the A549-FP3 model. This indicates that the median time required for the tumors to reach a volume of 1 cm3 is about twice as long when treated with A-1331852 as compared to a sham-treated control. When comparing the combination to the most effective single-agent treatment, the increase in amplitude and durability of the response was statistically significant (P < 0.05) in five of seven models. The effect was most pronounced in the MDA-MB-231 LC3 metastatic breast cancer model (Fig. 5A) and the NSCLC models NCI-H1650 (Fig. 5B) and NCI-H358 (Table 4). Overall, the single-agent and combination treatments were well tolerated by mice, without overt signs of toxicity or weight loss of >9%. These data demonstrate that BCL-XL inhibition alone can enhance the efficacy of docetaxel in a variety of solid tumor models.[1] In this study, researchers first embarked on in vivo efficacy studies where A-1331852 was dosed as monotherapy or in combination with docetaxel,13 the results of which have been reported recently. We additionally utilized A-1331852 to evaluate other combinations of BCL-XL inhibition with standard chemotherapy in vivo. We recently reported that BCL2L1 (BCL-XL) amplification characterizes a subset of colorectal cancer (CRC) cell lines, including the Colo205 cell line.16 Furthermore, in vitro knockdown of BCL-XL protein expression in CRC cells by antisense oligonucleotides can enhance the apoptotic response to topoisomerase I (Topo I) inhibitors,24 thereby suggesting a potential combination treatment therapy of a small-molecule BCL-XL inhibitor with a Topo I inhibitor such as irinotecan.Reference: ACS Med Chem Lett. 2020 Oct 8; 11(10): 1829–1836. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7549103/ To assess the potential of this combination in an in vivo setting, A-1331852 was assessed for efficacy in the Colo205 murine xenograft model of human colorectal cancer as single agent and in combination with irinotecan, as shown in Figure Figure55. SCID/Beige mice were inoculated with Colo205 cells and size-matched to tumor volumes of approximately 220 mm3, after which A-1331852 was dosed orally as a single agent or in combination with irinotecan. TGImax-values following treatment with A-1331852 (25 mg/kg/day, QD × 14) or irinotecan (30 mg/kg/day, Q3D × 4) were 35 or 75%, respectively. Treatment with a combination of A-1331852 and irinotecan resulted in a TGImax value of 92%. Thus, tumor growth inhibition following the combination treatment was significantly (p < 0.001) higher than after treatment with irinotecan alone. Furthermore, the combination also significantly (p < 0.001) increased the durability of the response (TGD = 254%) as compared to that observed after treatment with irinotecan (TGD = 162%) alone.Reference: ACS Med Chem Lett. 2020 Oct 8; 11(10): 1829–1836. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7549103/ |
| Enzyme Assay |
Binding Affinity Assays[1] Time-resolved fluorescence resonance energy transfer (TR-FRET) binding affinity assays were performed for BCL-2, BCL-XL, and MCL-1 as described previously for BCL-XL. Compounds were serially diluted in DMSO starting at 500 μM using a Tecan Gemini robot. An intermediate 1:10 dilution in assay buffer was performed using a Tecan Temo, and 10 μL transferred to a white 384-well low-volume Corning #3673 assay plate (2× starting concentration; 10% DMSO). Then 10 μL of a protein/probe/antibody mix was added to each well at final concentrations as follows: 1 nM GST-labeled protein, 1 nM Terbium anti-GST antibody and 100 nM Oregon green-labeled BAK peptide. The samples were then equilibrated for 1 h at room temperature. For each assay, probe/antibody and protein/probe/antibody were included on each assay plate as negative and positive controls, respectively. Time-resolved fluorescence was measured on an Envision plate reader with a 340 nm excitation filter and 520 nm (f-BAK) and 495 nm (Tb-labeled anti-His antibody) emission filters. Dissociation constants (Ki) were determined using Wang’s equation[1]. |
| Cell Assay |
Immunoprecipitation of BCL-XL is carried out in K562 cells, exposed to A-1331852 (100 nM) for 0-2 h, and the eluted complexes are immunoblotted for the indicated proteins. To assess the effectiveness of the immunoprecipitation, immunoblotting is done on both the input cell lysates and the immunodepleted supernatant (labeled as Flow-through). Cell proliferation and viability assays [1] Breast cancer cell lines were seeded at 5,000 cells per well in 96-well plates and treated with compound combinations in a 9×3 dose matrix, with navitoclax, venetoclax, and A-1155463 diluted in three-fold steps (20-0.001 μM) and docetaxel at 50, 5.0, or 0.5 nM. Cells were incubated for 72 h before assessing viability. NSCLC cell lines were treated for 72 h with compound combinations in a 5×5 dose matrix and assessed as described previously[1]. Ovarian cancer cell lines were seeded at 10,000 cells per well in 96-well plates and treated with compound combinations in a 9×3 dose matrix for 48 h. Docetaxel was diluted in three-fold steps (10-1.1 nM). Navitoclax, venetoclax, and A-1155463 were diluted in 2-fold steps (20-0.08 μM).[1] Colony-Forming Assays[1] Hematopoietic precursor cells derived from normal human bone marrow (BM) were incubated with various concentrations of navitoclax, venetoclax, or A-1155463 plus or minus 5 nM docetaxel in MethoCult 4230 methylcellulose-based medium supplemented with 30 ng mL-1 recombinant human granulocyte colony stimulating factor (rhGCSF). DMSO was used to make stock solutions of all test compounds and was present at a final concentration of <0.002% in all wells. Frozen BM light density cells from three different lots (BM07B21195, BM0080512A, and BM5H09) were thawed rapidly at 37°C, washed once in 10 mL Iscove’s Modified Dulbecco’s Medium (IMDM) supplemented with 2% fetal bovine serum (IMDM + 2% FBS), and resuspended in IMDM + 2% FBS. Between 2.4-4.3×104 viable cells were seeded in each well of 6-well plates and incubated at 37°C (5% CO2) in the presence of test compounds for 14-16 days. Colony-forming units comprising at least 30 granulocyte cells were enumerated by a trained technician using light microscopy. Each condition was tested in triplicate to determine mean colony numbers +/- one standard deviation. |
| Animal Protocol |
Mice: SCID-bg mice are used to study the tumors' ability to grow. A-1331852 is given intravenously at 7.5 mg/kg every day for 14 days, while RP-56976 is given orally at 25 mg/kg daily. Every day, the tumor's volume change is tracked. Compounds and Formulations [1] For administration in vivo, A-1331852 was formulated in 60% Phosal 50 PG, 27.5 % PEG 400, 10% ethanol, and 2.5 % DMSO. First, A-1331852 was suspended in DMSO and ethanol until a uniform cloudy suspension was obtained. PEG 400 and Phosal were then added and the solution was mixed by vortexing. Allowing the solution to sit for approximately 30 min after adding all the excipients helped to achieve a clear solution. A probe sonicator was also used for less than 10 min. Formulated compound was stored in an amber bottle at room temperature to protect it from light. A-1331852 was administered per os (PO) in this formulation. Docetaxel (DTX, Taxotere, Sanofi) in a solution of 50/50 (v/v) ratio polysorbate 80/dehydrated alcohol was diluted with saline prior to intravenous (IV) injection. When combined, DTX was given 1 h after A-1331852. Rat studies [1] Three separate rat studies were conducted using male Sprague Dawley rats (Crl:CD). Each study consisted of four arms, with 10 rats per arm: vehicle control PO daily for 5 consecutive days, docetaxel dosed IV as a single dose on day 1, BCL-XL, BCL-2, or BCLXL/ BCL-2 inhibitors dosed PO daily for 5 consecutive days, and BCL-XL, BCL-2, or BCLXL/ BCL-2 inhibitors in combination with docetaxel (docetaxel was dosed as a single dose on day 1, followed immediately by a dose of BCL-2 family inhibitor, and continued daily doses of inhibitor on days 2-5). Control rats were dosed with vehicle: 80% PEG/20% TPGS at 2 mL/kg for the study with BCL-XL inhibitor A-1331852, a 20/80 mixture of Vitamin E TPGS/PEG400 and 0.9% Phosphate Buffered Saline at 5 mL/kg for the study with BCL-2 inhibitor A-1211212, and 10% ethanol/30% PEG-400/60% Phosal 50 PG for the study with the BCL-2/BCL-XL dual inhibitor A-874009. In each study, rats were dosed with docetaxel as a single agent, via intravenous (IV) bolus at a dose of 5 mg kg-1 (10 mL kg-1 volume) or with the inhibitors as single agents at 7 mg kg-1 (2 mL kg-1 volume) of A-1331852, 50 mg kg-1 (5 mL kg-1 volume) of A- 1211212, or 30 mg kg-1 (5 mL kg-1 volume) of A-874009. For the combination dosing, an IV bolus of 5 mg kg-1 of docetaxel was immediately followed by a dose of 7 mg kg-1 of A-1331852, 50 mg kg-1 of A-1211212, or 30 mg kg-1 of A-874009. |
| References |
[1]. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med. 2015 Mar 18;7(279):279ra40. |
| Additional Infomation |
The BCL-2/BCL-XL/BCL-W inhibitor ABT-263 (navitoclax) has shown promising clinical activity in lymphoid malignancies such as chronic lymphocytic leukemia. However, its efficacy in these settings is limited by thrombocytopenia caused by BCL-XL inhibition. This prompted the generation of the BCL-2-selective inhibitor venetoclax (ABT-199/GDC-0199), which demonstrates robust activity in these cancers but spares platelets. Navitoclax has also been shown to enhance the efficacy of docetaxel in preclinical models of solid tumors, but clinical use of this combination has been limited by neutropenia. We used venetoclax and the BCL-XL-selective inhibitors A-1155463 and A-1331852 to assess the relative contributions of inhibiting BCL-2 or BCL-XL to the efficacy and toxicity of the navitoclax-docetaxel combination. Selective BCL-2 inhibition suppressed granulopoiesis in vitro and in vivo, potentially accounting for the exacerbated neutropenia observed when navitoclax was combined with docetaxel clinically. By contrast, selectively inhibiting BCL-XL did not suppress granulopoiesis but was highly efficacious in combination with docetaxel when tested against a range of solid tumors. Therefore, BCL-XL-selective inhibitors have the potential to enhance the efficacy of docetaxel in solid tumors and avoid the exacerbation of neutropenia observed with navitoclax. These studies demonstrate the translational utility of this toolkit of selective BCL-2 family inhibitors and highlight their potential as improved cancer therapeutics.[1] Cancerous inhibitor of protein phosphatase 2A (CIP2A) is a predictive biomarker of disease progression in many malignancies, including imatinib-treated chronic myeloid leukemia (CML). Although high CIP2A levels correlate with disease progression in CML, the underlying molecular mechanisms remain elusive. In a screen of diagnostic chronic phase samples from patients with high and low CIP2A protein levels, high CIP2A levels correlate with an antiapoptotic phenotype, characterized by downregulation of proapoptotic BCL-2 family members, including BIM, PUMA and HRK, and upregulation of the antiapoptotic protein BCL-XL. These results suggest that the poor prognosis of patients with high CIP2A levels is due to an antiapoptotic phenotype. Disrupting this antiapoptotic phenotype by inhibition of BCL-XL via RNA interference or A-1331852, a novel, potent and BCL-XL-selective inhibitor, resulted in extensive apoptosis either alone or in combination with imatinib, dasatinib or nilotinib, both in cell lines and in primary CD34(+) cells from patients with high levels of CIP2A. These results demonstrate that BCL-XL is the major antiapoptotic survival protein and may be a novel therapeutic target in CML.[2] |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: 2.08 mg/mL (3.16 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.08 mg/mL (3.16 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (3.16 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: ≥ 2.5 mg/mL (3.79 mM) (saturation unknown) in 2.5% DMSO 10% ethanol 27.5% PEG 300 60% Phosal 50 PG (add these co-solvents sequentially from left to right, and one by one), clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5179 mL | 7.5894 mL | 15.1789 mL | |
| 5 mM | 0.3036 mL | 1.5179 mL | 3.0358 mL | |
| 10 mM | 0.1518 mL | 0.7589 mL | 1.5179 mL |