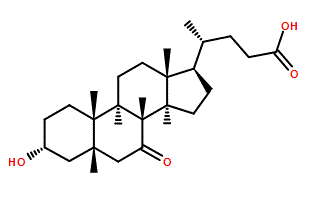
7-Ketolithocholic acid (Nutriacholic Acid; 3α-Hydroxy-7-oxo-5β-cholanic acid) is a Lithocholic Acid derivative that can be absorbed in the intestine, thus suppressing endogenous bile acid production and biliary cholesterol secretion.
Physicochemical Properties
| Molecular Formula | C₂₄H₃₈O₄ |
| Molecular Weight | 390.56 |
| Exact Mass | 390.277 |
| CAS # | 4651-67-6 |
| PubChem CID | 444262 |
| Appearance | Typically exists as White to off-white solid at room temperature |
| Density | 1.1±0.1 g/cm3 |
| Boiling Point | 545.9±25.0 °C at 760 mmHg |
| Melting Point | 205ºC |
| Flash Point | 298.0±19.7 °C |
| Vapour Pressure | 0.0±3.3 mmHg at 25°C |
| Index of Refraction | 1.535 |
| LogP | 4.25 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Heavy Atom Count | 28 |
| Complexity | 645 |
| Defined Atom Stereocenter Count | 9 |
| SMILES | C[C@H](CCC(O)=O)[C@H]1CC[C@@]2([H])[C@]3([H])C(C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)=O |
| InChi Key | DXOCDBGWDZAYRQ-AURDAFMXSA-N |
| InChi Code | InChI=1S/C24H38O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-19,22,25H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17-,18+,19+,22+,23+,24-/m1/s1 |
| Chemical Name | (4R)-4-[(3R,5S,8R,9S,10S,13R,14S,17R)-3-hydroxy-10,13-dimethyl-7-oxo-1,2,3,4,5,6,8,9,11,12,14,15,16,17-tetradecahydrocyclopenta[a]phenanthren-17-yl]pentanoic acid |
| Synonyms | 7-Ketolithocholic acid; 7 Ketolithocholic acid; 4651-67-6; 7-Ketolithocholic acid; Nutriacholic acid; 3alpha-Hydroxy-7-oxo-5beta-cholanic acid; 7-Oxolithocholic acid; 3.alpha.-Hydroxy-7-oxo-5.beta.-cholanic acid; 3a-Hydroxy-7-oxo-5b-cholanic acid; 3alpha-Hydroxy-7-oxo-5beta-cholan-24-oic Acid; Nutriacholic Acid; 3α-Hydroxy-7-oxo-5β-cholanic acid |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Endogenous Metabolite |
| ln Vitro | The primary components of bile are bile acids. They are processed steroids that the liver tissue produces from bile [1]. |
| ln Vivo | The effect of 7-ketolithocholic acid on biliary bile acid composition, cholesterol saturation, and as an intermediate in the conversion of chenodeoxycholic acid to ursodeoxycholic acid was investigated in 5 subjects with gallstones. After 7-ketolithocholic acid (400 mg/day) was administered orally for 14 days, biliary bile acid composition changed: The proportion of cholic acid decreased (from 45% to 19%), deoxycholic acid decreased (from 15% to 10%), chenodeoxycholic acid increased markedly (from 36% to 59%), ursodeoxycholic acid increased (from 36% to 59%), ursodeoxycholic acid increased (from 2% to 7%), and lithocholic acid increased (from 2% to 5%), while only trace amounts of 7-ketolithocholic acid were detected. During this treatment, the biliary lithogenic index fell from 2.6 to 0.9 and was accompanied by a pronounced drop in biliary cholesterol concentration. After biliary bile acid levels became constant [24-14C]chenodeoxycholic acid was given intravenously as a pulse-label, and the resultant biliary ursodeoxycholic acid and lithocholic acid specific activity curves showed a precursor--product relationship with chenodeoxycholic acid. Similarly, when uniformly labeled 7-[24-14C]ketolithocholic acid was fed (400 mg/day, 1000 +/- 100 dpm/mg) the specific activities of biliary chenodeoxycholic acid and ursodeoxycholic acid became constant and approximated each other, but these were only 75% as high as the fed 7-ketolithocholic acid. These results indicate that 7-ketolithocholic acid is absorbed, and suppresses endogenous bile acid production and biliary cholesterol secretion. Both isotopic experiments infer that ursodeoxycholic acid and lithocholic acid are formed from chenodeoxycholic acid and not from 7-ketolithocholic acid. The reduction in biliary lithogenic index and in cholesterol concentration suggest that low doses of 7-ketolithocholic acid may be effective in dissolving gallstones.[2] |
| References |
[1]. Quantification of bile acids: a mass spectrometry platform for studying gut microbe connectionto metabolic diseases. J Lipid Res. 2020 Feb;61(2):159-177. [2]. Effect of 7-ketolithocholic acid on bile acid metabolism in humans. Gastroenterology. 1982 Aug;83(2):341-7. |
| Additional Infomation |
7-oxolithocholic acid is a bile acid that is lithocholic acid carrying an additional oxo substituent at position 7. It has a role as a human metabolite. It is a bile acid, a monohydroxy-5beta-cholanic acid, an oxo-5beta-cholanic acid and a 3alpha-hydroxy steroid. It is functionally related to a lithocholic acid. It is a conjugate acid of a 7-oxolithocholate. Bile acids (BAs) serve multiple biological functions, ranging from the absorption of lipids and fat-soluble vitamins to serving as signaling molecules through the direct activation of dedicated cellular receptors. Synthesized by both host and microbial pathways, BAs are increasingly understood as participating in the regulation of numerous pathways relevant to metabolic diseases, including lipid and glucose metabolism, energy expenditure, and inflammation. Quantitative analyses of BAs in biological matrices can be problematic due to their unusual and diverse physicochemical properties, making optimization of a method that shows good accuracy, precision, efficiency of extraction, and minimized matrix effects across structurally distinct human and murine BAs challenging. Herein we develop and clinically validate a stable-isotope-dilution LC/MS/MS method for the quantitative analysis of numerous primary and secondary BAs in both human and mouse biological matrices. We also utilize this tool to investigate gut microbiota participation in the generation of structurally specific BAs in both humans and mice. We examine circulating levels of specific BAs and in a clinical case-control study of age- and gender-matched type 2 diabetes mellitus (T2DM) versus nondiabetics. BAs whose circulating levels are associated with T2DM include numerous 12α-hydroxyl BAs (taurocholic acid, taurodeoxycholic acid, glycodeoxycholic acid, deoxycholic acid, and 3-ketodeoxycholic acid), while taurohyodeoxycholic acid was negatively associated with diabetes. The LC/MS/MS-based platform described should serve as a robust, high-throughput investigative tool for studying the potential involvement of structurally specific BAs and the gut microbiome on both physiological and disease processes.[1] |
Solubility Data
| Solubility (In Vitro) | DMSO : ≥ 100 mg/mL (~256.04 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.40 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (6.40 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5604 mL | 12.8021 mL | 25.6043 mL | |
| 5 mM | 0.5121 mL | 2.5604 mL | 5.1209 mL | |
| 10 mM | 0.2560 mL | 1.2802 mL | 2.5604 mL |