Physicochemical Properties
| Molecular Formula | C14H8O3 |
| Molecular Weight | 224.21 |
| Exact Mass | 224.047 |
| Elemental Analysis | C, 75.00; H, 3.60; O, 21.41 |
| CAS # | 129-43-1 |
| PubChem CID | 8512 |
| Appearance | Yellow to orange solid powder |
| Density | 1.4±0.1 g/cm3 |
| Boiling Point | 414.8±14.0 °C at 760 mmHg |
| Melting Point | 194°C |
| Flash Point | 218.8±16.6 °C |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.695 |
| LogP | 3.97 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Heavy Atom Count | 17 |
| Complexity | 349 |
| Defined Atom Stereocenter Count | 0 |
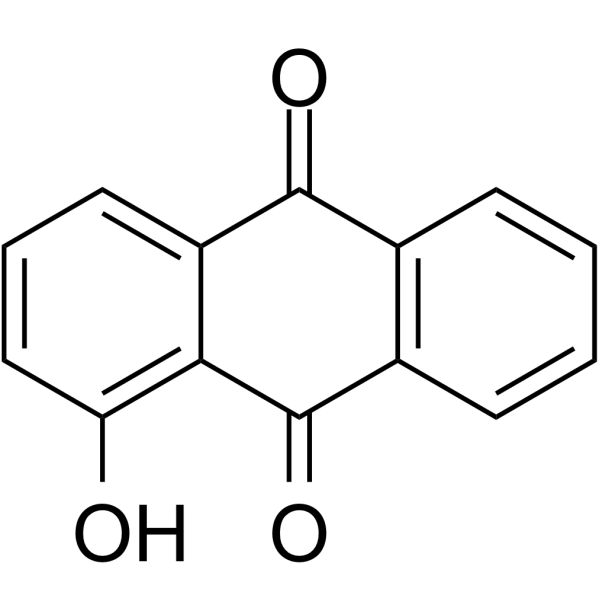
| SMILES | C1=CC=C2C(=C1)C(=O)C3=C(C(=CC=C3)O)C2=O |
| InChi Key | BTLXPCBPYBNQNR-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C14H8O3/c15-11-7-3-6-10-12(11)14(17)9-5-2-1-4-8(9)13(10)16/h1-7,15H |
| Chemical Name | 1-hydroxyanthracene-9,10-dione |
| Synonyms | 1-Hydroxyanthraquinone; NSC 8640; NSC-8640; NSC8640; 1-HYDROXYANTHRAQUINONE; 129-43-1; 1-hydroxyanthracene-9,10-dione; 1-Hydroxy anthraquinone; 9,10-Anthracenedione, 1-hydroxy-; 1-Hydroxy-9,10-anthraquinone; 1-Hydroxyanthrachinon; Hydroxyanthraquinone; 1 Hydroxyanthraquinone |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Naturally occurring anthraquinone |
| ln Vitro | 1-Hydroxyanthraquinone has been isolated from the roots of Rubia cordifolia, Morinda officinalis and Damnacanthus indicus, from the heartwood of Tabebuia avellanedae and the herb Cassia occidentalis. |
| ln Vivo |
1-Hydroxyanthraquinone (HA) produces a potent response for DNA repair and is carcinogenic in rats[1]. The carcinogenic potential of 1-hydroxyanthraquinone (HA), a naturally occurring compound, was examined. A total of 60 male ACI/N rats, 1.5 months old at the commencement were divided into two groups. Group 1 (30 rats) were fed the diet containing HA at a concentration of 1% throughout the experiment (480 days). Group 2 (30 rats) served as the control given a basal diet alone. Twenty-five of 29 effective animals in group 1 developed adenomas or adenocarcinomas in the cecum or upper portion of the colon, the mean number of large bowel tumors/tumor bearing rat being 2.3. In addition to these intestinal tumors, liver neoplasms (neoplastic nodules and hepatocellular carcinomas) were observed in 12 rats and benign stomach tumors were obtained in five animals; no rats of group 2 demonstrating development of any of these tumor types. The incidences of the large bowel, liver and stomach neoplasms in group 1 were all significant as compared with group 2 (P less than 2 x 10(-13), P less than 5 x 10(-5) and P less than 3 x 10(-2) respectively) clearly indicating that HA is carcinogenic in rats.[1] |
| Animal Protocol |
Animal/Disease Models: Thirty rats[1]. Doses: 1% HA in diet. Route of Administration: Diet. Experimental Results: Associated with diminished weight gain which was particularly marked towards the termination of experiment. One of the 30 rats in group 1 (experimental group) died of pneumonia 243 days after the start of experiment. A second rat died in an unnourished state at day 280, demonstrating a large tumor in the colon. Seven animals of the group died spontaneously or were sacrificed upon becoming moribund between 335 and 462 days. A total of 21 rats of group 1 survived until the end of experiment (mean value of total intake of HA/rat was 76.8 g). |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion 1-Hydroxyanthraquinone was found to be absorbed when continuously administered to rats (strain not specified) by stomach tube (50 mg suspended in aqueous gum arabic), and its metabolites were identified by paper chromatography. Urine and feces were collected during 48 hours after treatment and extracted with diethyl ether. Of the 1-hydroxyanthraquinone originally present, 2.49% and 0.74% were converted into alizarin (1,2-dihydroxyanthraquinone), in urine and feces, respectively. The alizarin was then excreted after sulfation and glucuronidation. Metabolism / Metabolites ... 1-Hydroxyanthraquinone was found to be absorbed when continuously administered to rats (strain not specified) by stomach tube (50 mg suspended in aqueous gum arabic), and its metabolites were identified by paper chromatography. Urine and feces were collected during 48 hours after treatment and extracted with diethyl ether. Of the 1-hydroxyanthraquinone originally present, 2.49% and 0.74% were converted into alizarin (1,2-dihydroxyanthraquinone), in urine and feces, respectively. The alizarin was then excreted after sulfation and glucuronidation. |
| Toxicity/Toxicokinetics |
Interactions ... In addition, 1-HAQ has a synergistic effect with methylazoxymethaol (MAM) acetate on colon carcinogenesis. |
| References |
[1]. Carcinogenicity of naturally occurring 1-hydroxyanthraquinone in rats: induction of large bowel, liver and stomach neoplasms. Carcinogenesis. 1990 May;11(5):799-802. |
| Additional Infomation |
1-Hydroxyanthraquinone can cause cancer according to California Labor Code. 1-hydroxyanthraquinone is a monohydroxyanthraquinone. 1-Hydroxyanthraquinone has been reported in Rheum palmatum, Morinda citrifolia, and Handroanthus impetiginosus with data available. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~16.7 mg/mL (~74.4 mM) |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.4601 mL | 22.3005 mL | 44.6010 mL | |
| 5 mM | 0.8920 mL | 4.4601 mL | 8.9202 mL | |
| 10 mM | 0.4460 mL | 2.2301 mL | 4.4601 mL |